5.3.3 Proton Transfer Reaction Ionization (PTR)
PTR is a relatively recent addition to mass spectrometry (1995) that was originally developed for GC and LC, there is not reason that it can not be used for CE. It was developed at the Institut für Ionenphysik at the Leopold-Franzens University in Innsbruck, Austria by Hansel et al. (1995). As shown in Figure 5.8, the PTR consists of a reaction chamber where water vapor is ionized to gas phase ions by hollow cathode discharge via the following reactions
![]()
These products undergo ion-water vapor reactions in a short drift tube to form
![]()
The hydronium ion (H3O+) is end product and the primary reacting ion that ionizes organic analytes in the reaction drift tube via the reaction
![]()
Unlike in TOF or ion mobility MS, reaction ions are not subjected to a electrical potential in the drift tube but are moved through the system by placing a low pressure vacuum pump at the interface of the PRT drift tube and the inlet to the mass filter (refer to Figure 5.8). Analyte cations created in the drift tube enter a mass filter where they are separated by the operating parameters of each mass filter and are detected with an electron mulitiplier.
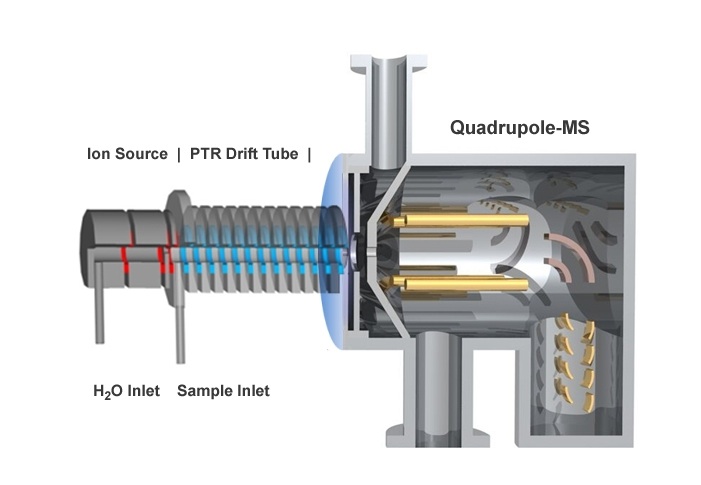
Figure 5.8 Illustration of a Proton Transfer Reaction – MS System. Reprinted with permission from Ionicon Analytik Gesellschaft, Innsbruck, Austria.
A PTR-MS is illustrated via the link in Animation 5.4.
http://www.uibk.ac.at/ionen-angewandte-physik/umwelt/research/pics/animation.gif
Animation 5.4. Illustration of a Proton Transfer Reaction—Mass Spectrometer.
Advantages of the PTR-MS include (1) low fragmentation with allows improved detection limits due to the formation of more molecular ions, (2) direct sampling of atmospheric gases (no sample preparation), (3) real time measurements, (4) high mobility due to the lack of gas cylinders, relative ease of operation only requiring electrical power and distilled water, and part per billion detection limits.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008