6.11.4 Fragmentation of Aldehydes
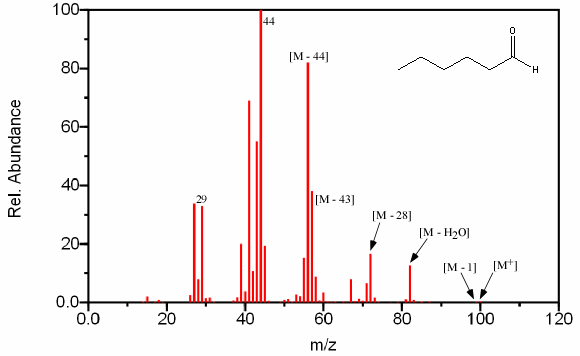
The major peaks observed in spectrums of aldehyde are the result of the same α cleavage as in ketones. This fragmentation results in an [M – 1] peak and a peak at [M – R] from the COH+ ion. The presence of an [M – 1] peak helps to identify the aldehyde but the [M – R] peak (m/z 29) is not unique to this type of compound. Compounds that contain carbon and hydrogen atoms frequently produce a complex hydrocarbon rearrangement at m/z 29 (C2H5). Another prominent peak is the McLafferty rearrangement located at m/z 44. The only aldehydes that do not contain this peak are ones that lack the necessary hydrogen atom for this rearrangement.
Straight chain aldehydes have unique features that help in identification. These compounds will have a [M – 18] fragment from the loss of water, [M – 28] from the loss of ethylene, [M – 43] loss of CH2=CH–O and [M – 44] from the loss of CH2=CH–OH (Figure 2.16).

Figure 6.16 Fragmentation of an Aldehyde. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
The patterns resulting from aromatic ketones are almost identical to those governing aromatic aldehydes. The characteristic molecular ion is accompanied by a [M – 1] peak from the loss of hydrogen. The ArC≡O fragment loses CO to form the phenyl ion at m/z 77 that further degrades to give a peak at m/z 51.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008