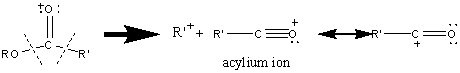
6.14 Fragmentation of Esters
The molecular ion peak of straight chain esters is sometimes discernable. A prevalent peak and often the base peak results from the familiar McLafferty rearrangement. The size of the alcohol that formed the ester and the presence of α substituents can normally be discerned by the mass of these two peaks. Esters also under go a cleavage of the bond α to the carbonyl group.
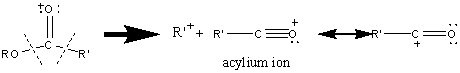
The cleavage of the above bonds results in other fragments, however these peaks are too small to be of great significance. For example, hexanoic acid methyl ester produces the following fragments.
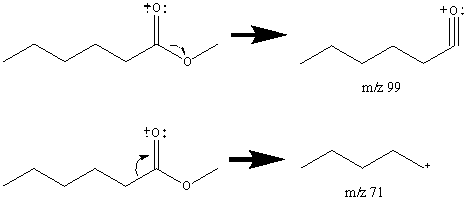
The resonance stabilized acylium ion gives a discernable peak for almost all esters. The R’+ ion is prominent in short chain esters but is barely visible in esters with more than six carbon atoms. For hexanoic acid methyl ester, the R’+ ion is only 9.5% of the base peak.
Until this point, this chapter has covered individual functional groups in isolation. Since esters have both an alcohol and an acid component, fractionation patterns can be observed from both of these types of compounds. The prevalence of the fragments described earlier is dependent on the size of each part of the ester. The increased size of each portion results in a unique rearrangement.
When the acid portion is the major component like in hexanoic acid methyl ester, the fractionation pattern is partially characterized by typical acid peaks. For these straight chain esters, cleavage of successive carbon atoms gives an alkyl fragment and a fragment containing oxygen. This pattern results in the familiar grouping of fragments spaced 14 units apart with the largest fragment in the cluster resulting from the CnH2n-1O2 ion (Figure 6.19).
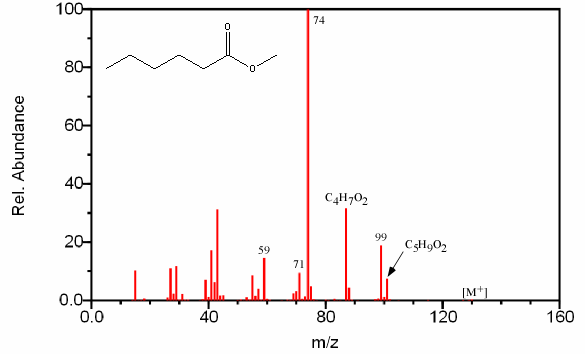
Figure 6.19. Fragmentation of an Ester. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
The base peak in Figure 6.19 is a result of the McLafferty rearrangement.
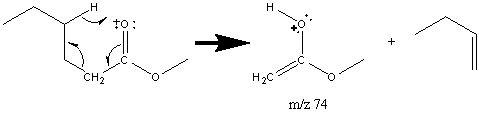
When the alcohol portion of the ester is the prominent portion of the ester, fragments similar to that of an alcohol are observed. These esters will lose a molecule of acid like alcohols loose a molecule of water. In esters formed from larger alcohols, the prevalence of this rearrangement is so frequent that the molecular ion is normally absent from the spectra.
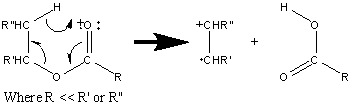
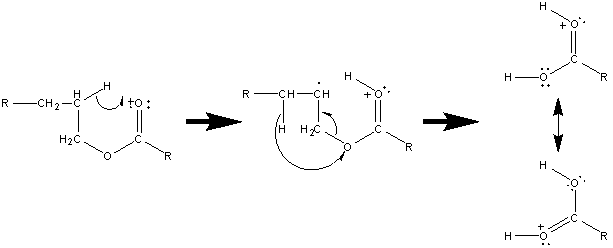
These types of esters will also lose the alkyl fragment from the alcohol portion of the ester accompanied by two hydrogen migrations.
In aromatic esters, the molecular ion is prominent due to the aromatic group. There are two distinctive types of aromatic esters that have their own unique fractionation patterns. Esters synthesized from aromatic acids mostly undergo α cleavages.
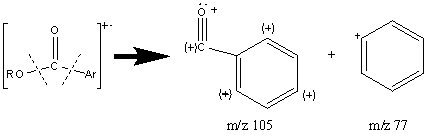
The loss of ·OR results in the base peak because of the multiple resonance forms stabilizing the cation. When the alkyl portion of the alcohol becomes longer, the McLafferty rearrangement and the loss of R with two hydrogen migrations explained above is more favorable. Increasing the size of the R chain will cause the alkyl portion to retain the charge.
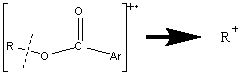
Esters synthesized from aromatic alcohols have a prominent peak at m/z 43 due to the CH3C≡O+ ion. These esters also undergo a rearrangement that results in the loss of a ketene molecule.
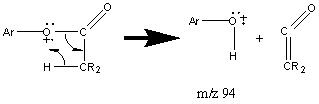
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008