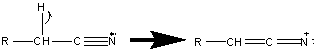
6.17 Fragmentation of Nitriles
The presence of the nitrogen can usually be identified by the odd molecular weight according to the nitrogen rule (Section 6.3). This identification technique is usually unable to identify a nitrile because these compounds lack a molecular ion. The presence of a [M – 1] peak complicates the identification of the molecular ion. This peak is formed by a loss of an α hydrogen to form a resonance stabilized cation.
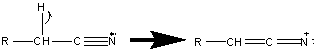
A prominent and frequent base peak is the result of the McLafferty rearrangement at m/z 41 in compounds whose α carbon is not branched. This peak, however, is unable to confirm that a compound is a nitrile because hydrocarbon chains frequently form a peak at C3H5.
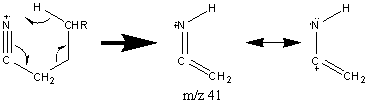
A unique peak at m/z 97 is characteristic of nitriles that contain a straight chain of seven carbons or more.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008