6.9.2 Fragmentation of Branched Alkanes
Branched alkanes have a smaller molecular ion, that at times, may be absent in highly branched compounds. In larger compounds branched alkanes contain peaks at CmH2m+1, similar to straight chain alkanes. They are distinguished by the lack of the smooth exponential decay beginning at the C3 or C4 carbon (Figure 6.9). This is caused by preferential fragmentation at the branch site, resulting in a more stable secondary carbocation. The loss of the largest alkyl fragment at the branched site is favored because it helps to stabilize the radical.
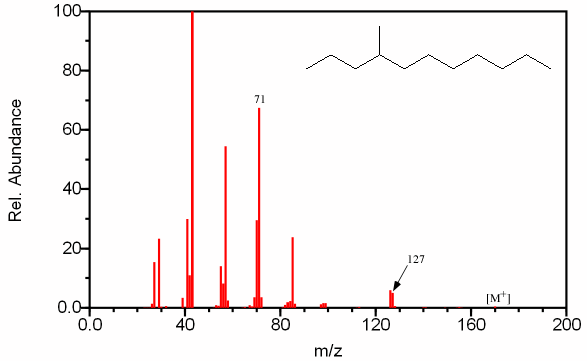
Figure 6.10. Fragmentation of a Branched Alkane. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
This mass spectrum of a C12 alkane (determined from the molecular ion by chemical ionization at m/z 170) lacks the exponential decay seen in Figure 6.9 indicating the chain is branched. The intensity of the peak at m/z 71 indicates a favored C5 fragment and the fragment at m/z 127 indicates a favored C9 fragment suggesting that a methyl group is located on the fourth carbon.
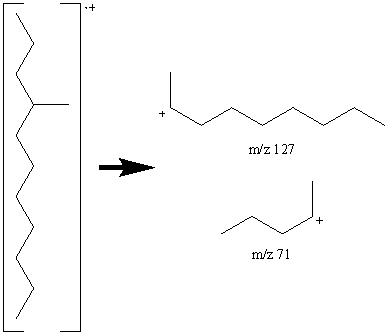
The fragmentation at the branching point is often accompanied by hydrogen rearrangement causing the CnH2n peak to be more prominent and sometimes even larger than CnH2n+1 peak.
Identifying branched alkanes in organic compounds that contain another functional group is also an important task. The alkane portion of these molecules is usually smaller and is governed by the stability of the produced radical and cation. Since an ethyl radical is more stable than a methyl radical, the methyl radical occurs less frequently. Similarly, tertiary carbocations are more stable than secondary, which are more stable than primary. As the alkane portion of any molecule becomes larger, the presence of the CnH2n+1
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008