1.2.2 Relaxation Processes
The absorption of a photon by an atom or molecule results in the creation of an excited energy state. Given that this atom or molecule would prefer to exist in the ground state, the energy that was absorbed is quickly dissipated by various pathways of relaxation, or (rarely) by breaking a bond. While the instruments that are described in this Etextbook produce atomic species from molecular ones with a variety of heat sources, they do not use electromagnetic radiation for atomization (the creation of gaseous). Instead they produce atomic species from organic or inorganic molecules in a flame or plasma. Nonradiative relaxation, emissions, fluorescence, and phosphorescence are all types of relaxations that occur without breaking a bond. For purposes of clarity, it should be noted that while some of these relaxations are not analytically useful for the techniques described here, they are of importance in other spectroscopic techniques. Some of these are only relevant to molecular spectrometry, while others are relevant to both atomic and molecular spectrometry. For completeness, all three will be described in this text.
Nonradiative relaxations are not completely understood, but occur through the transfer of very small amounts of energy through molecular or atomic collisions. These result in the generation or transfer of heat energy, but do not cause the emission of a UV or visible photon. Emission of a photon that has the same amount of energy as the absorbed photon is also a very common form of relaxation.
Fluorescence is a relatively rare form of relaxation in polyatomic compounds. Fluorescence occurs when an analyte is excited to a higher energy state by the absorption of a photon. The molecule then undergoes a vibrational relaxation (again, in polyatomic compounds) to a lower vibrational state within the same electronic state. Fluorescence emission occurs when the electron falls from one excited electronic state back to the ground electronic state (a singlet to singlet transition). This type of transition occurs since most vibrational transitions occur faster (10-15 to 10-12 seconds) than electronic transitions (around 10-8 seconds). Since the vibrational relaxation emits energy in the form of heat, the energy of the fluorescence emission is lower (and consequentially the wavelength is longer) than the absorbed energy. This is illustrated in Figure 1-4. Measurements of fluorescence usually yield detection limits that are from one to three orders of magnitude more sensitive than typical absorption or emission detection limits. The lower detection limits are due to lower background noise, since the exciting radiation is not detected in the analytical measurement.
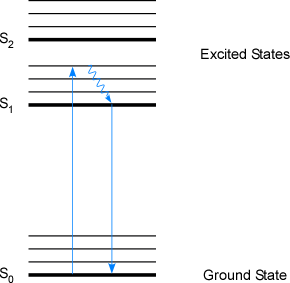
Figure 1-4. A Jablonski Diagram Illustrating Fluorescence Decay in a Molecular Species. (Bold lines are electronic transition states while lighter shaded lines represent vibrational transitions)
Phosphorescence occurs when a radiationless vibrational transition occurs between two different spin states (for example from a singlet (S1) to a triplet (T1) excited state). After this spin forbidden transition, another spin forbidden transition from the excited state to the singlet state must occur (from a triplet to singlet state in Figure 1-5). Phosphorescence transitions are easy to distinguish from fluorescence since they occur much more slowly; fluorescence occurs over time scales of 10-5 seconds while phosphorescence occurs over time scales of minutes to hours since spin forbidden transitions have a low probability of occurring (a low transition rate).
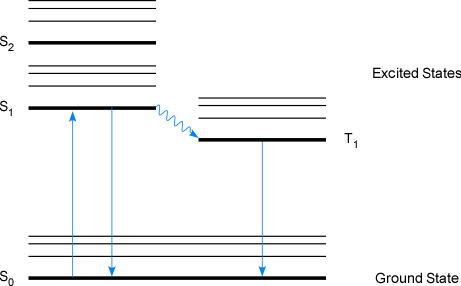
Figure 1-5. A Jablonski Diagram Illustrating Phosphorescence Decay in a Molecular Species.
In molecular spectroscopy fluorescence and phosphorescence instruments, the incident (source) radiation never reaches the detector. Instead the detector aperture is orthogonal (90 degrees off set) to the source lamp’s path in order to reduce background noise from the incident source light. Source light is focused through a sample and generally passes straight through, with the exception of scattered radiation. Fluorescence and phosphorescence are emitted from the sample at all angles, so orientation of the detector at 90 degrees minimizes the source light scattered to the detector. A very useful technique is possible with phosphorescence, since emission can still be detected after the source lamp is turned off. It is possible to place a shutter between the excited sample and the detector. The shutter is opened after the source lamp is turned off and phosphorescence is occurring. This allows the detector to measure only phosphorescence. Phosphorescence has no use in atomic spectrometry, but can be used for the analysis of metals in polyatomic states, such as those occurring for uranium oxide species.
For quantification of fluorescence (F) and phosphorescence (P) of polyatomic species, Beer’s law is slightly modified to
F or P = I Φ ε b C
where I is the intensity of radiation passing through the sample, Φ is the yield of fluorescence or phosphorescence (or how much of the source radiation is converted to fluorescence or phosphorescence), ε is molar extinction coefficient, b is the cell path width, and C is the molar concentration.
Other forms of fluorescence are possible, but they involve molecular vibrational relaxations that are not relevant to atomic species. One common type of florescence is referred to as “resonance fluorescence”. This occurs when an electron in the ground state is excited to a higher energy state by a photon whose energy is nearly resonant with the transition energy. This is followed by the emission of a photon of the same energy as the electron relaxes (basically a normal absorption/emission process; this is the same type of emission that was discussed earlier). Emission of a photon from resonance fluorescence almost always occurs in absorbance spectroscopy, but is usually not relevant because the fluorescence intensity is relatively weak in comparison to the intensity of the light source. Two other types of florescence occur that cause the emitted photon to have less energy than the absorbed photon. ”Direct line fluorescence” is when an electron is excited to an upper energy level (S3) and emits a photon to go to a lower energy level (S2) that is not the ground state. “Stepwise line fluorescence” occurs when an electron is excited to a higher energy state (S3), and relaxes to S2 by some form of nonradiative relaxation, followed by a photon emission to return to the ground state.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008