3.3 Components of an Inductively Coupled Plasma—Atomic Emission Spectrometry System (ICP-AES)
3.3.2 Sample Introduction and Optimization
The predominate form of sample matrix in ICP-AES today is a liquid sample: acidified water or solids digested into aqueous forms. Given the automated nature of the ICP analysis, all modern systems are purchased with automatic samplers where a computer-controlled robotic sampling arm takes liquids from each sample via a peristaltic pump from plastic tubes located in specific locations in a sampling tray. Liquid samples are pumped into the nebulizer and sample chamber via a peristaltic pump as shown below. Then the samples pass through a nebulizer that creates a fine mist of liquid particles. Larger water droplets condense on the sides of the spray chamber and are removed via the drain (pumped out of the chamber also by the same peristaltic pump) while finer water droplets move with the argon flow and enter the plasma. Nebulizers help ensure that the sample enters into the plasma at a uniform flow rate and specific droplet size. Droplets that are great than 5 µm in diameter are likely to interfere with plasma stability.
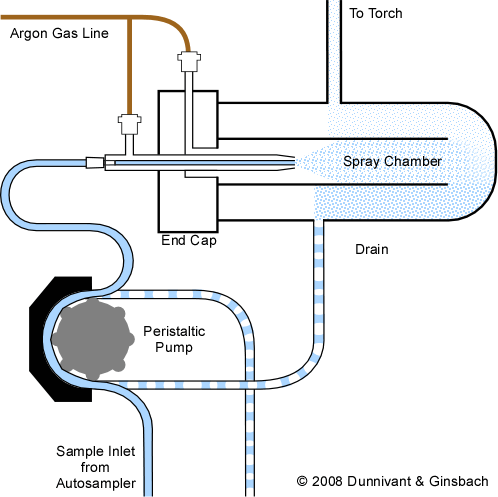
Figure 3-2. An Overview of Sample Introduction and the Nebulizer Chamber. (The nebulizer shown here is a pneumatic style, described below.)
While there are numerous types of nebulizers for a variety of specific applications, the three most commonly types are the (1) pneumatic, (2) ultrasonic, and (3) grid. Because argon is used in generating the plasma (discussed below) it is most often used as the gas in these various nebulizers, but other gases can be used. The most common pneumatic nebulizer for samples containing low concentrations of total dissolved solids is the concentric nebulizer shown in Figure 3-3, but higher suspended solids and dissolved solids samples are commonly introduced to the plasma via the Babington nebulizer.
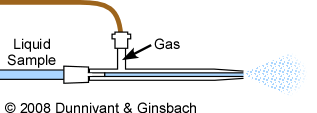
Figure 3-3. Diagram of a Pneumatic Concentric Nebulizer.
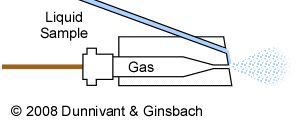
Figure 3-4. Diagram of a Pneumatic Babington Nebulizer.
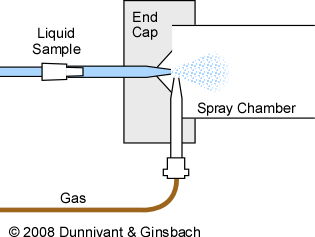
Figure 3-5. Diagram of a Pneumatic Cross-Flow Nebulizer.
Ultrasonic nebulizers are used to provide more sample delivery to the plasma and thus improve detection limits. An ultrasonic generator surface, usually a piezoelectric crystal that rapidly vibrates to generate sonic energy, is used to create extremely fine droplets that, at low flow rates, are completely transferred to the plasma (unlike considerably lower efficiencies from pneumatic nebulizers). Grid nebulizers create a fine mist by placing a grid in front of the argon flow. The liquid sample is allowed to flow down the grid and as argon passes through the grid it creates fine droplets. Again, the most common commercially available nebulizers use the pneumatic design.
ICP systems have a few sample requirements or limitations. Micrometer-sized particles must be removed by filtration or centrifuge or dissolved prior to introduction to a sample vial because the particles can easily clog the tip of the nebulizer. A clogged tip will not be automatically detected by the instrument, but will be detected by a trained operator by the absence of any sample, internal standard, or standard analyte concentrations in the final.
Another limitation of the nebulizer is the presence of high total dissolved solids (TDS) that will eventually cause the accumulation of solids in the tip of the nebulizer, as well as in the sample chamber and on the sampler cones located just after the plasma (in ICP-MS instruments only). Most ICP instruments limit the sample TDS level to approximately 0.1 to 0.2 percent salts by weight. Higher salt contents can enhance atomization and ionization of some elements and suppress or interfere with others (Walsh, 1997). Likewise, the acid concentration of liquids entering the plasma should be limited to approximately two to three percent for nitric and hydrochloric acid. Rarely hydrofluoric acid may be used if a particularly refractory element is of interest, but then the acid concentrations are less than 0.5% because it will degrade the quartz torch with time. Some samples, such as food-based beverages or plant extracts, can contain high concentrations of organic matter. It is best to oxidize this organic matter in an acid or peroxide digestion prior to analysis since carbon will rapidly accumulate on the components of the instrument if high organic matter containing samples are directly injected into the plasma. This carbon can reduce sample flow and capture elements and ions as the carbon builds up, specifically at the cone apertures that facilitate the transition from ambient pressure in the plasma region to the high vacuum detector region (in ICP-MS systems). When a portion of a carbon plaque drops back into the analytical stream, an anomalously high, and false, measurement can occur.
Other forms of sample introduction are used but are not as common as liquid injection. These include (1) direct insertion of 10-30 mg of sample on a graphite probe into the plasma via the normal nebulizer entry port, (2) the injection of the effluent from chromatographic separation systems, especially from high performance liquid chromatography (HPLC), supercritical chromatography, and ion chromatography (IC) units where compounds containing elemental analytes of interest are first separated by chromatography and then introduced into the plasma for elemental analysis, (3) where the cold vapor techniques described in Section 2.4 for mercury analysis is connected to the plasma inlet via the nebulizer port, and (4) where the hydride generation system from Section 2.4 for selected metals can also be connected to the nebulizer port.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008