2.2.2 Liquid Samples
Liquid samples are the next easiest to analyze by GC since they are already in an injectable matrix. Samples from organic synthesis procedures usually have products (analytes) present at high concentrations and are analyzed by direct injection. Unfortunately, relatively few products fit the requirements of GC—that analytes be volatile and thermally stable—so most products are analyzed by HPLC, the subject of Chapter 3. Analytes in aqueous samples are also easy to analyze by GC. Some GC detectors and columns allow the direct injection of aqueous samples if the concentration of the analyte is sufficiently high. The aqueous sample is frequently extracted into an organic solvent using a standard separatory funnel when there is a low concentration of analyte is present in sample or when water could harm the GC column or detector. Usually the aqueous sample is extracted three times with a relatively small volume of organic solvent, the organic extracts are combined, the volume is reduced by evaporation, and the resulting organic extract is injected into the GC. One disadvantage of the organic extraction is the need to purchase expensive and very pure organic solvents (typically priced at approximately $150 for four liters) and the expensive disposal costs of the resulting organic waste solvents. A more automated version of the separatory funnel is a liquid-liquid extractor, but the glassware is relatively expensive and typical extraction times run from 8 to 24 hours. In this extraction setup, the organic solvent is boiled, condensed, and passed through a water vessel where the non-volatile hydrophobic analytes partition into the organic solvent that is constantly recycled into the boiling vessel. The recycled solvent is re-evaporated again, leaving the analytes in the boiling flask, and passes again into the condenser and water column. Figure 2.1 shows a typical liquid-liquid extraction system.
 |
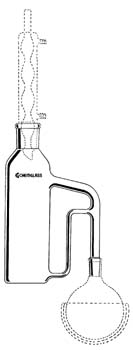 |
Figure 2.1 A Liquid-Liquid Extractor for Extracting Analytes from Water Samples. Reprinted with permission from VWR Scientific Products International, Chemglass Life Sciences, and Corning.
A relatively easy way to avoid the need for expensive glassware is to use resin packs (SPE; solid phase extraction) that are available from a variety of vendors. In this technique, the water sample is passed through a resin pack (a solvent-resistant tube usually one to a few centimeters in diameter and slightly taller in height). Again, the resin has a high affinity for the analytes. After the passage of the aqueous sample through the packet, the resin is dried by passing ultra-pure gas through it and the adsorbed analytes are removed by passing a small volume of organic solvent (usually a few mL) through the packet. The solvent volume is adjusted to a known volume and injected into the GC.

Figure 2.2 Three Resin Packets for Extracting Analytes from Aqueous Samples.
An even more novel way of extracting analytes from water samples is to use Solid Phase Micro Extractors (SPMEs) that consist of a syringe containing a fused silica capillary fiber coated with a chromatography stationary phase with a high affinity for the analytes of interest. The fiber is housed in a metal needle where it can be extended for collecting analytes or for desorption in a GC injection inlet. The SPME needle and fiber are passed through a septum in the sample bottle, either into the gaseous space above the water or directly into the water, the fiber is exposed through the end of the needle and allowed to equilibrate (adsorb the analytes) for typically 10 to 30 minutes while the sample is mixed with a stir bar. After this time most or all of the analytes are transferred to the SPME fiber. The fiber is drawn into the metal needle; the needle is withdrawn from the sample bottle and placed directly into the GC injector. The advantages of this technique are (1) no need for expensive organic extraction solvents, (2) relatively rapid analysis, (3) possibly improved extraction recovery, and (4) significant concentration of the analytes and improvement of detection limits (up to 10 000 to 1 000 000 fold concentrations). Fibers can be reused from 50 to 100 times. The minor disadvantage of the SPME technique is the cost of the apparatus (approximately $600 for three fibers and a holder/injector).
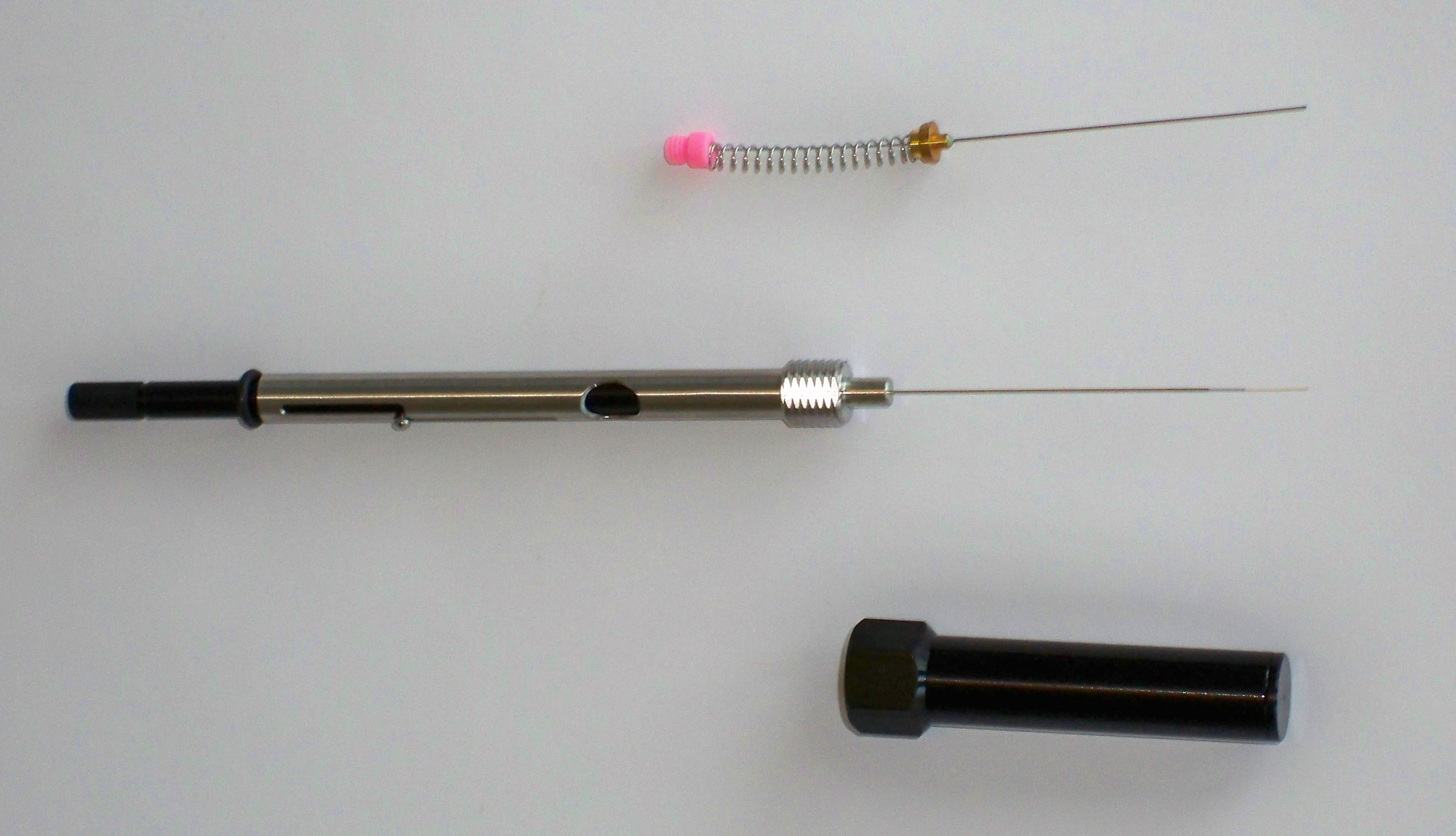
Figure 2.3 A SPME Device with the Microfiber Exposed (middle item). Extra needles are available (top item) since the injector syringe can be reused indefinitely. Needles can be reused from 50 to 100 times depending on the composition of the sample. The bottom item is the needle protection guard and GC injection guide.
Volatile analytes present an additional problem since considerable quantities of the analyte can be lost during sample preparation. Analyses of volatile analytes are best preformed with some type of commercial head-space analyzer or “purge and trap” device where the actual water sample (with no gaseous headspace) is attached to a sample processing unit, a gas is used to transfer the volatile analytes to a resin trap or directly into the GC injector, and after the required purge time the transferred analytes are analyzed by GC.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008