3.1 Introduction and History
In Chapter 2, Michael Tswett (1872-1920) was credited as the father of chromatography due to his 1903 separation of the green-leaf pigments into bands of colors, a demonstration of liquid chromatography. While similar work was being conducted in the petroleum industry, Tswett is credited with coining the term “chromatography”. Despite Tswett’s results, chromatography did not develop quickly. The next major developments were the use of thin-layer chromatography (TLC) in 1937-38 and the use of paper chromatography in the mid-1940s, but thin layer chromatography quickly won popularity. Thin layer chromatography was originally developed by Nikolai Izmailov (1907-1961) and his graduate student Maria Shraiber (1904-1992) for pharmaceutical preparations. Early TLC was conducted with microscope slides that were coated with suspensions of calcium, magnesium, and aluminum oxides. As used today, a small spot of solution was placed on one end of the slide, the slide was dipped into a solvent, and the analytes migrated at different rates through the oxide coatings where they were later detected (today by a UV lamp or chemical stain).
TLC advanced slowly during the next few years but a major advancement was made in 1956 by Egon Stahl (1924-1986) when he attempted to standardize the preparation of the sorbents used to make the plates. While these advances and others such as forced flow TLC, significantly matured TLC into an accepted (and reproducible) practice; it was still only a qualitative technique, at best. However, Izmailov and Shraiber’s spot chromatography, commonly known today as TLC, is the workhorse of undergraduate organic synthesis labs where synthesis reactions are conducted and the resulting products are selected for using common glass open columns filled with silica gel (refer to Figure 3.1). Eluent from these columns is collected in fractions that are then run by TLC to identify which column fraction contains the desired product.
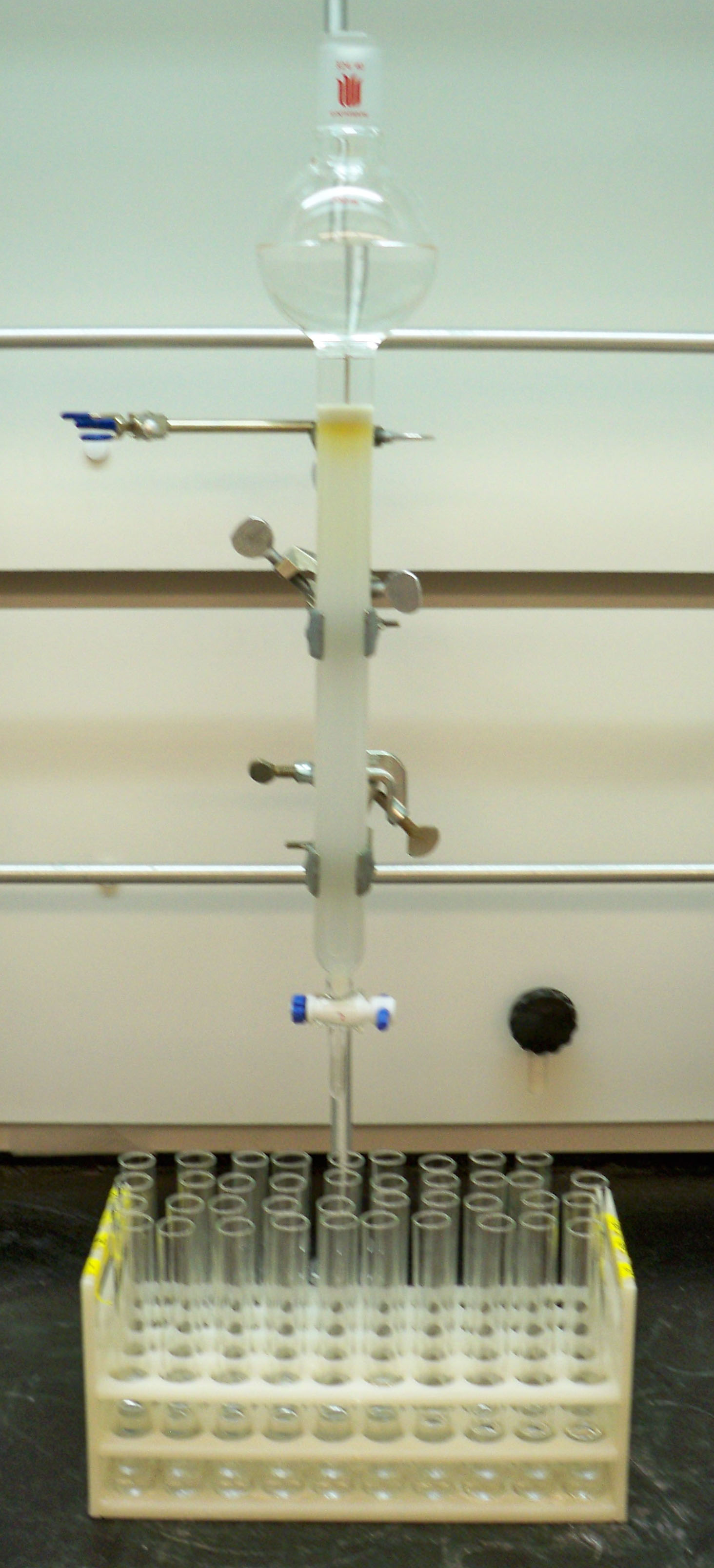
Figure 3.1 An Atmospheric Pressure Open Column Chromatographic Column. Fractions are collected in the test tubes and later ran by TLC to determine the purity of the fraction and the presence of synthesis products.
Since the development of TLC, liquid chromatography needed a technique comparable to gas chromatography where a complex mixture of analytes could be quantitatively separated and identified. Several attempts were made to pressurize the relatively large glass preparatory column (shown above) with little success due to the fragile nature of the column. High-pressure liquid chromatography (HPLC) was later developed to meet this goal in the 1970s. The pressure was first delivered by a large syringe, but this approach limited the volume of solvent that would be passed through a column and therefore limited the analysis time. Syringes were later replaced by a single reciprocating pump but these delivery systems experienced flow surges, between strokes of the single piston, interfering with stable detector baselines. The placement of two reciprocating pump, operating opposite to each other with respect to flow, greatly minimized the flow fluctuations which were later removed completely with a pulse damper. This form of chromatography is referred to as HPLC, where the HP stands for high performance or high pressure. Some jokingly refer to the HP as meaning high priced since it replaced TLC plates, that only cost pennies, with $20 000 to $30 000 instruments. The inflation of the cost of a LC analysis is even greater when an HPLC-MS is considered, a minimum of $150 000. But regardless, HPLC-MS is considered the technique of choice for isolating a synthetic product and is widely utilized in most synthesis laboratories.
Chromatography, as noted in Chapter 1, is divided into gas, liquid and supercritical fluid techniques. Liquid chromatography can be divided up into a relatively large collection of techniques. Those mentioned above include atmospheric or low-pressure open column chromatography and thin layer chromatography. Pressurized liquid chromatography can be divided into ion exchange, exclusion, partition, and liquid-solid chromatography as summarized in Animation 3.1. View this animation by double clicking on the figure below.
Animation 3.1. A Discussion of the Various Types of Chromatography.
As noted in Animation 3.1, a variety of separation techniques are available with high pressure liquid chromatography. Most relevant to this chapter is partition chromatography, although a few others will be discussed in later sections. The most important point here is to distinguish between normal and reverse phase chromatography. HPLC was first developed using normal phase conditions (NP-HPLC), that followed the logic of atmospheric open-column chromatography, where the stationary phase acted as the polar phase and the mobile phase was non-polar, specifically an organic solvent. Normal phase HPLC focused on the separation of analytes that were readily soluble in non-polar solvents but had slight affinities for the polar stationary phase. However, NP-HPLC required the use of relatively expensive and large volumes of organic solvents that also led to high waste disposal costs. NP-HPLC was effectively replaced by reverse phase HPLC (RP-HPLC) that operates with a non-polar stationary phase and an aqueous, moderately polar mobile phase. Gradient programming, an additional development, changes the composition of the mobile phase during a chromatographic run which greatly enhanced the utility of RP-HPLC. In RP-HPLC, the gradient is initially more polar (i.e. water) and as the chromatographic run progresses, more and more less-polar solvent is added to the mixture (i.e. methanol or acetonitrile) to end the gradient program with pure organic solvent. As noted in Animation 3.2, the retention order of analytes in RP-HPLC is opposite NP-HPLC. For example, the first analyte that eludes on a RP system would elude last is separated by NP-HPLC.
Animation 3.2 Demonstration of Normal and Reverse Phase HPLC.
Additionally, the pH of the polar solvent in RP-HPLC can play an important role in optimizing analyte separations. Ionic or ionizable analytes that would not normally be separated on a RP (non-polar) analytical column can also be analyzed by ion-pair chromatography. In this type of chromatography, the ionic analyte is bound to another ion (usually a large organic counter-ion such as quaternary ammonium or alkyl sulfonate) to form a neutral pair that has selective affinity for the non-polar stationary phase. Even many chiral compounds can be separated on special chiral stationary phases or by the additional of chiral resolving agents that selectively bind to one of the enantiomers. Additional modifications to an HPLC system such as ultra-high pressure LC, ion exchange, and supercritical fluid chromatography will be discussed in Sections 3.5 and 3.6.
One final point should be made with respect to HPLC. Chemists use HPLC for two completely different purposes. Organic chemists, especially in the pharmaceutical industry, use large-scale systems, referred to as preparatory HPLC or flash chromatography, to recover relatively large-scale milligram quantities of their products. The main differences of a preparatory HPLC, as opposed to an analytical HPLC, are the pump flow rates and the size of the columns. In contrast, analytical chemists use HPLC to separate and identify nanogram or smaller quantities of analytes. Which ever practice is needed, the overall chromatography is the same.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008