3.3.7 Columns
Guard Column:
The next component of an HPLC is the guard column. Guard columns are miniature versions of the analytical (separation) column and they contain the same stationary phase. The purpose of the guard column is to adsorb any permanently adsorbing chemicals that could destroy the more expensive analytical column. Guard columns typically cost one-fourth or less of the cost of an analytical column.
Analytical Column:
The heart of an HPLC system is the analytical column. This is where the mixture of chemicals injected in the system is separated into individual analytes that appear as peaks in the chromatogram. Columns are available in a variety of diameters and lengths ranging from large preparatory columns (20 - 50 mm in diameter by 50 - 250 mm in length), to analytical columns (typically 4.5 mm in diameter by 12 - 25 mm in length), to narrow bore analytical columns for improved performance and MS applications (1-2 mm in diameter by 10 cm in length), to capillary columns for MS detectors (from 0.075 - 0.1 mm in diameter). Larger columns require a larger mobile phase volume to push the analytes through the system. A collection of HPLC columns is shown in Figure 3.5.

Figure 3.5 Various Sizes of HPLC Analytical and Quard Columns. Source: Analytical Sales and Services, Inc. Reprinted with permission from Analytical Sales and Services.
With the exception of relatively new capillary columns, all HPLC columns are packed with small resin beads (typically 2-5 μm in diameter or less) that contain a coating of stationary phase. As in GC, stationary phases are selected based on the expected intermolecular forces available to the analyte for bonding. The adage “like dissolves like” is also appropriate for liquid chromatography applications. Separations in HPLC are considerable more complicated then in GC. In GC, the chromatographer is only concerned with the stationary phase and the temperature program. In HPLC, one must also be concerned with the polarity of the mobile phase. Adequate analyte separations are only achieved with an appropriate match of gradient polarity and stationary phase interactions. In more advanced applications, the pH and ionic composition of the mobile phase must also be controlled. The most common stationary phases used in reverse-phase HPLC include alkylamine, octodecyl (C18), octyl (C8), butyl (C4), cyanopropyl (CN), and methyl functional groups. Additional and custom-made stationary phases can be ordered from many suppliers.
Early chromatography columns that were open to the atmosphere were easy to pack. The chromatographer filled a glass column (1 to 5 cm in diameter) with solvent, slowly poured in silica gel with stirring to remove air pockets, and applied the solution to be separated to the top of the column. These types of columns yielded adequate separation for organic chemists attempting to isolate their synthesis products but have extremely limited use for analytical chemists who require more theoretical plates in the column and on-line detection of column effluents. Today, analytical chromatographers almost exclusively purchase their separation columns from manufactures because of the need for perfectly packed columns with no void spaces. The emphasis here is on perfect column packing since the presence of only a few void areas in a column will significantly affect the theoretical plate height in the column and decrease resolution. A comparison of a poorly packed column and an adequately packed column is shown in Animation 3.5.
Animation 3.5 The Effect of Adequate Column Packing on Peak Broadening in HPLC Columns.
The particle size of the stationary phase also significantly affects the operation of an HPLC by determining the shape of the van Deemter curve. Figure 3.6 shows the van Deemter curves for a variety of particle-sized stationary phases. Recall from our discussions in Chapter 1, better separations occur at the minimum point (linear velocity) in the van Deemter curve and a more stable system operation will occur with a large range of linear velocities. In HPLC, smaller sized stationary phase particles will yield a van Deemter curve that is essentially flat (refer to Figure 3.6). Thus, a range of flow rates will produce optimum analyte separation.
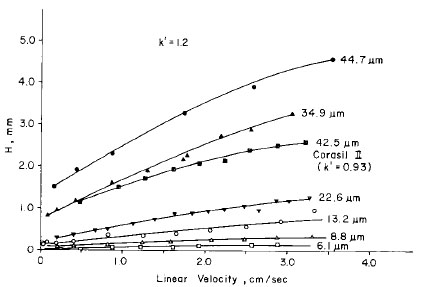
Figure 3.6 The Effect of Stationary Phase Particle Size on the Optimum Flow Rate in HPLC. From R.E. Majors, Effect of Particle Size on Column Efficiency in Liquid-Solid Chromatography, 1973, 11, 88. Reproduced from the Journal of Chromatographic Science by permission of Preston Publications, A Division of Preston Industries, Inc.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008