3.4 Advanced and Specialty LC Systems
3.4.2 Ion Chromatography (IC)
Ion chromatography is a form of HPLC where the system is modified to analyze for mainly inorganic ions such as nutrient ions (i.e. nitrate, sulfate, phosphate, etc.) as well as many organic anions and metal cations. The systems require a special ion exchange analytical column that is specific to the analytes of interest, a relatively simple conductivity detector, and a unique ion suppressor column for removing ions other than the analytes that would generate electrical conductivity in the detector. Columns and tubing are usually composed of PEEK to avoid reactions on metal surfaces. Analytical columns are mainly divided into columns for cations and anions and are similar in diameter to standard HPLC columns but slightly longer in length. Cationic exchange columns have active strong acid sites such as sulfonic acid (-SO3-H+) or weak acid sites such as carboxylic acid groups (-COO-H+) that preferentially exchange with analyte cations. Common anionic exchange sites include the strongly basic tertiary amines (-N(CH3)+OH-) and the weakly basic primary amine group (-NH3+OH-). Both exchange groups are usually placed on porous microparticles of silica. As the injected sample passes through the analytical column, shown below for the sulfonic acid exchange surface, the following reaction occurs
-R-SO3-H+SOLID + Metal Cation+SOLUTION < --- > (-R-SO3-)nMetal Cation+SOLID + nH+SOLUTION
Similarly for anion analysis, the reaction would be
-NH3+OH-SOLID + Anion-SOLUTION < --- > (-NH3+)nAnion-SOLID + nOH-SOLUTION
At appropriate flow rates, an equilibrium migration front is set up for each analyte passing through the column. Since each metal cation will have a different affinity for the stationary ion exchange resin, each analyte will elute from the column at a different time. As in all types of chromatograph (in the absence of mass spectrometry detection), identification is largely based on retention time.
The detector in IC is a simple conductivity detector where a potential is placed across two electrodes. In the absence of ions in the solution passing through the detector, minimal current is transmitted and no signal is generated by the electronics. As each packet of analytes, cations or anions, pass through the detectora current is generated that is proportional to the concentration of analytes. In order to detect low concentrations of analytes, the background signal (current) of the mobile phase must be maintained as low as possible. Since all samples have counterions (an equal concentration of cations or anions) half of the ionic components must be removed prior to entering the detector. For example, detecting the presence of cations require the removal of anions before the column effluent reaches the detector. The removal of these ions is accomplished with an ion suppressor device shown in Figure 3.7. This device is positioned within the ion chromatography system as shown in Figure 3.8.

Figure 3.7 Photograph of Two Ion Suppressor Devices.
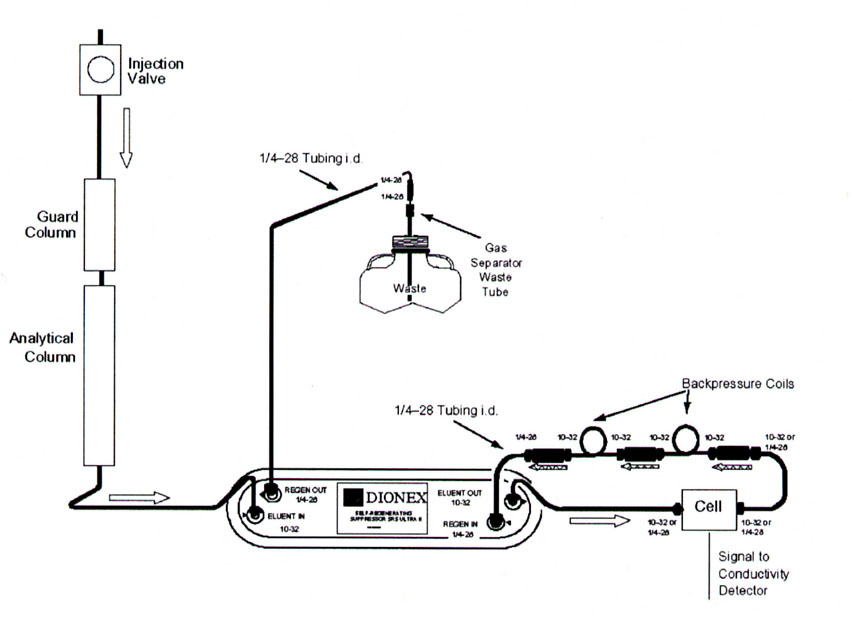
Figure 3.8 Overview of the Components used to Separate Analytes in an Ion Chromatography System. Source: Dionex Corporation Product Manual ASRS 300 CSRS 300, Figure 7, page 20. Reprinted with permission, courtesy of Dionex Corporation, Sunnyvale, California.
As presented in Figure 3.8, the sample first passes through the analytical column where the cations or anions are separated relative to their affinity for the solid-phase exchange resin. The effluent from the analytical column then enters the ion suppressor device, along with acid or base to keep the ion suppressor column active. For anionic analysis, strong acid (H+) is used in the suppressor device; for cationic analysis, strong base (OH- or -COO-) is used in the device. In both cases the analytes of interest are unchanged and unretained. As a consequence, the following suppression reactions occur depending on the mode of operation (cation or anion analysis).
In anion analysis, cations in the sample are exchanged for H+ in the suppressor column and neutralized in the center flow through portion of the suppressor column by the following reaction
H+SOLUTION + Cl-SOLUTION + resin+OH-SOLID --- > resin+Cl-SOLID + H2O
Thus, only anions, such as Cl-, contribute to the conductivity in the detector.
In cation analysis, anions in the sample are exchanged with HCO3- (or OH-) in the suppressor column and neutralized in the center flow through portion of the suppressor column by the following reaction
cation+SOLUTION + HCO3-SOLUTION + resin-H+SOLID --- > resin-cation+SOLID + H2CO3 SOLUTION
Thus, only cations, such as Ca2+, contribute to the conductivity in the detector.
Again, note that these reactions convert all ionic species, except for the analytes, to non-conductive chemicals. These reactions in relation to the flow through the ion suppressor are illustrated in Figure 3.9. In both cases, non-ionic species are produced and the interfering ions are removed (suppressed) from the solution. This significantly decreases the background ions in solution and allows for low detection limits (parts per trillion to parts per billion in the injected sample). Ion chromatography systems also operate well with MS detectors.
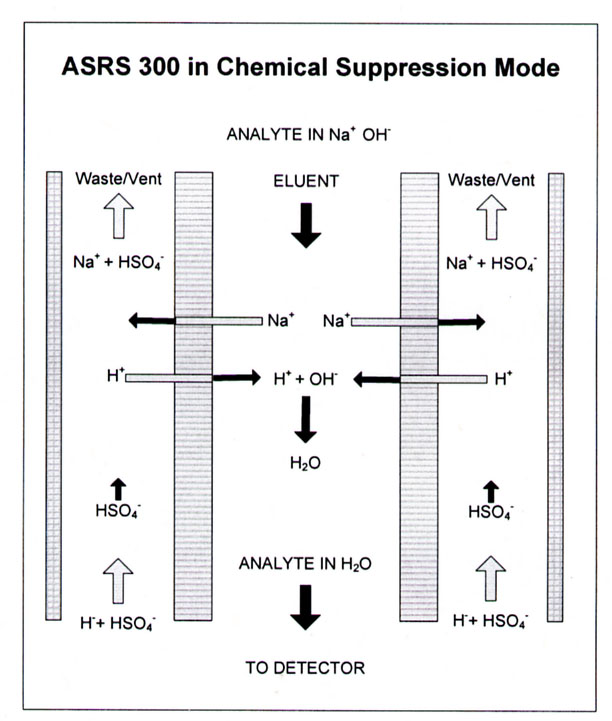
Figure 3.9 Cation suppression in an ion suppression device. Source: Dionex Corporation Product Manual ASRS 300 CSRS 300, Figure 4, page 9. Reprinted with permission, courtesy of Dionex Corporation, Sunnyvale, California
©Dunnivant & Ginsbach, 2008