4.4.2 Miceller Electrokinetic Chromatography (MEKC)
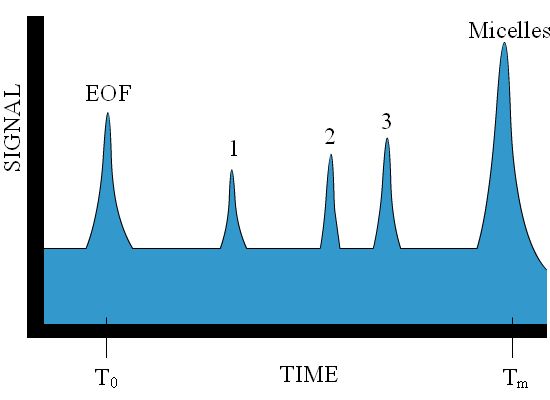
Miceller electrokinetic chromatography is a combination of electrophoresis and chromatography. If one dissolves surfactants (for example, 8-9mM SDS) in the running buffer, micelles--often anionic--form and travel against the EOF. The EOF, however, is still faster and the net motion of the micelles is in the same direction as the EOF. Solutes partition between the micelles and the buffer through hydrophobic and electrostatic interactions. In this way, the micelles act as a stationary phase which is not necessarily stationary, but slower with respect to the EOF. The more hydrophobic compounds migrate more slowly through the capillary due to their interactions with the micelles. All neutral compounds will elute between the EOF, which elutes first, and the micelles, which elute last. (See Figure 4.8).
The surfactants can be anionic, cationic, non-ionic, zwitterionic, mixtures of these, salts, or microemulsions. Deciding which surfactant to use is based on the specific interactions necessary to separate target compounds.

Figure 4.8. Example miceller electrokinetic chromatogram.
Surfactants may affect the EOF and solute-wall interactions. Generally, using a high pH buffer will maintain the EOF and diminish changes in solute-wall interactions. Additionally, a consistent temperature is especially important as changes in temperature will affect the concentrations of surfactant required to form micelles as well as the partition coefficient(s) of the system.