5.2.1.2 Ionization Techniques for Volatile Analytes Entering the MS from a GC
5.2.1.2a Electron Ionization or Electron Impact (EI):
Electron ionization of analytes is referred to as a hard ionization technique since it causes bonds to be broken within a sample molecule (fragmentation). Neutral, radical, and positively charged species are produced from fragmentation. Neutral and radical species are not affected by the accelerator plates or mass analyzer and are removed by the vacuum. Positive ions are accelerated towards the mass analyzer and some either (1) collide with a surface in the source (typically the accelerator plate) or (2) enter into the mass analyzer through the slit in the electronic lens. The ions that collide with any surface are neutralized and removed by the vacuum. The ions that enter into the mass analyzer are separated by mass to charge ratios. The high degree of fragmentation can be an advantage in compound identification. When more ion fragments are created, the more unique the fragmentation pattern, and the more confirmatory analyte identification will be. On the other hand, the detection of the molecular ion in EI can be difficult, which is often a goal of MS analysis in organic chemistry.
Electron ionization works by forcing the stream of pure analytes exiting the GC through a beam of high energy electrons in the MS. Electrons are created by heating a metal filament, usually tungsten, to a temperature high enough to expel electrons. Electrons are drawn toward an anode, passing through the stream of analyte molecules. It is important to note that electrons do not actually impact analyte molecules as implied by the name “electron impact”. The high energy of the electron (70 eV) is actually transferred to an analyte when the electronic transition of the analyte matches the frequency of the electron. The exact electron energy was selected through experimentation. It was found that a 70 eV electron energy source resulted in the most reproducible spectra and in a high degree of fragmentation. This 70 eV condition is now the standard and all computer libraries of fragmentation are based on this energy level.
The animation below shows a beam of electrons that is generated by a heated filament at the bottom of the figure that is accelerated toward the anode at the top of the figure. When different analytes (in this case butane) exit the GC column (the brown column on the left) and cross through the electron beam, an electron from the sample molecules is removed. Once the molecular ion is formed, they are forced to the right by repulsion from a positively charge accelerator plate on the left (not shown) and drawn toward the negatively charged accelerator plate to the right. Some butane molecules also fragment into smaller ions. The prevalence of this process is underestimated by the animation due to space restraints. The molecular ion and fragments would next enter the mass analyzer (shown later).
Animation 5.1. Illustration of an Electron Impact Chamber.
After the energy transfer between the electron beam and the analyte, the energy causes the molecule to become unstable and frequently cleave bonds. The fragmentation patterns are predictable because the types of bond cleavages a molecule undergoes is related to its structure (Chapter 6). The ionization rate is predicted to be between one in a thousand to one in a million of the molecules entering the ionization chamber. This level of successful ionization should be noted since MS detection limits are approximately one part per million and below (injected analyte concentration). In early systems, the instrument only ionized and detected approximately one millionth of the number of molecules that were injected; today this has been improved to about one in a thousand or more. Two examples of EI spectra are shown in Figures 5.1 and 5.2; note the extensive fragmentation of each analyte.
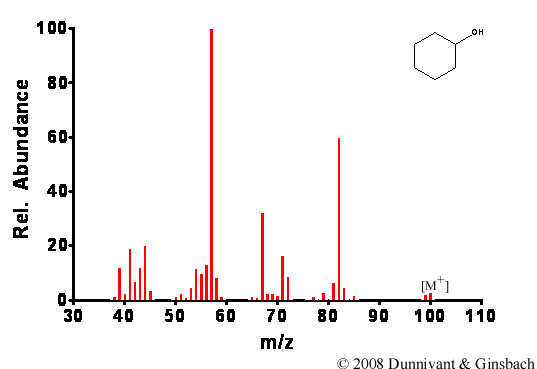
Figure 5.1. Fragmentation of Cyclohexanol by EI.
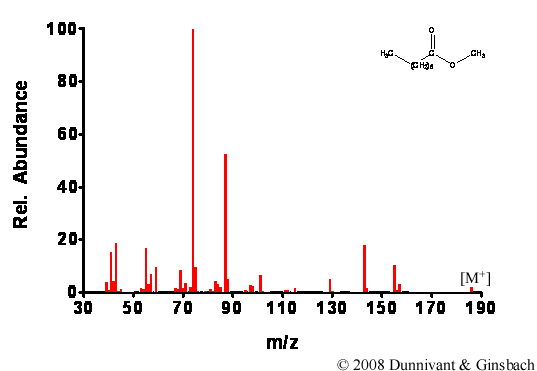
Figure 5.2. Fragmentation of Decanoic Acid Methyl Ester by EI.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008