5.2.1.2 Ionization Techniques for Volatile Analytes Entering the MS from a GC
5.2.1.2b Chemical Ionization (CI):
Today, most mass spectrometers can perform both electron ionization and chemical ionization, with different interchangeable ionization units. The CI unit is less open to diffusion of the reagent gas in order to contain the reagent gas longer and promote chemical ionization. Several reagent gases are used including methane, propane, isobutane, and ammonia, with the most common being methane. CI is referred to as a soft ionization technique since less energy is transferred to the original analyte molecule, and hence, less fragmentation occurs. In fact, one of the main purposes of using CI is to observe the molecular ion, represented by M.+ or M.-, or a close adduct of it, such as MH+, MH+2, or M plus the chemical ion (i.e. M+CH3 with methane as the reagent gas or M+NH3 with ammonia as the reagent gas). Notice again that neutral, negative, and positive fragments are produced but only the positive fragments are of use in positive CI detection, while negative ion fragments are detected in negative CI mode.
This section will limit its discussion to CI and methane, the most common reagent gas. Methane enters the ionization chamber at about 1000 times the concentration of the analyte molecules. While the electron beam in EI is usually set at 70eV, in CI lower energy levels are used near the range of 20 to 40 eV. This energy level produces electrons that react with methane to form CH4·+, CH3+, and CH2·+. These ions rapidly react with unionized methane in the following manner:
The CH5+ and C2H5+ ions collide with the analytes (represented by M) and form MH+ and (M-1)+ by proton and hydride transfer
Note that several types of ions can occur, (M+1)+ or MH+ from proton transfer, (M-1)+ from hydride transfer, and M+CH3+ and even M+C2H5+ from additions. By inspecting the mass spectrum for this pattern, the molecular mass of the analyte can be deduced. Similarly, if other reagent gases are used, such as propane, isobutene, and ammonia, similar proton and hydride transfer and adduct formations can occur. The usual goal of CI is to obtain a molecule weight for the molecular ion that would usually not be present in an EI spectra.
A relatively simple illustration of a CI chamber and its reactions is shown in the animation below. This animation is similar to the EI animation, but the continuous addition of a reagent gas, methane, causes the gas to be ionized by the beam of electrons. Subsequently, the ionized methane reacts with analytes exiting the GC column. Methane is preferentially ionized by the beam of electrons due to its significantly higher concentration as compared to analytes from the GC. Positively charged fragments are drawn into the focusing lens and mass analyzer by a positively charged repeller plate (not shown) and the negatively charged accelerator plate.
Animation 5.2. Illustration of a CI Chamber and Reagent Gas-Analyte Reactions.
Chemical ionization is most commonly used to create positive ions, but some analytes, such as those containing acidic groups or electronegative elements (i.e. chlorinated hydrocarbons) will also produce negative ions that can be detected by reversing the polarity on the accelerator and detector systems. Some of these analytes produce superior detection limits with CI as opposed to EI, while others only give increased sensitivity (slope of the response to concentration line). Negative ions are produced by the capture of thermal electrons (relatively slower electrons with less energy than those common in the electron beam) by the analyte molecule. Thermal electrons are present from the low energy end of the distribution of electrons produced by the lower-energy CI source (~20 eV as opposed to 70 eV in EI). These low energy electrons arise mostly from the chemical ionization process but also from analyte/electron collisions. Analyte molecules react with thermal electrons in the following manner, where R-R’ is the unreacted analyte molecule and R represents an organic group.
The identification of negative ion fragmentation patterns of analytes can be used in the same manner as in EI or positive ion CI. But note that extensive fragmentation libraries exist only for 70eV electron ionization (EI). Many analysts create their own reference libraries with the analysis of reference materials that will later be used for the identification of unknown analytes extracted from samples.
Figures 5.3 and 5.4 contain CI spectra for the same compounds analyzed by EI in Figure 5.1 and 5.2, respectively. Note the obvious lack of fragmentation with the CI source and the presence of molecular ions in the CI spectra.
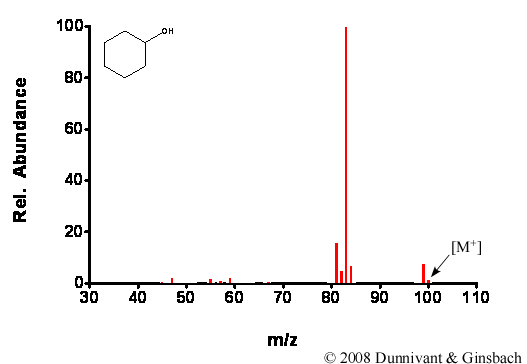
Figure 5.3. Fragmentation of Cyclohexanol by CI.
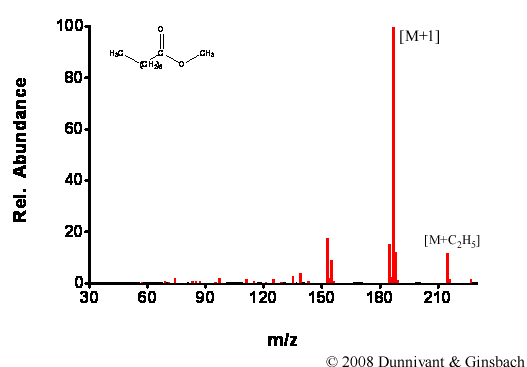
Figure 5.4. Fragmentation of Decanoic Acid Methyl Ester by CI.
To summarize, for GC-MS systems, individual analytes exit the GC column, are ionized, and fragmented using electron or chemical impact (ionization). Since the detector in a MS is universal (responds to any positively charged ion) it is necessary to separate the molecular ion and its fragments by their mass or mass to charge ratio. This process is completed in a mass analyzer, which is explained in the section below. But first, some mass analyzers require the beam of ion fragments to be focused and all require the ion fragments to be accelerated in a linear direction.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008