5.3.1 Electro-Spray Ionization (ESI) Sample Introduction
Today, the most common form of LS-MS interface is the ESI sample introduction system. An overview of this system is shown in Figure 5.6. Samples can be introduced via a syringe or an HPLC system (convention or capillary column type). A restriction in the syringe needle or HPLC column causes the solvent containing the analytes to form droplets. An electrical potential, discussed in the next paragraph, is placed between the sample inlet and the first cone. This cone separates the sample introduction from the vacuum chamber in the MS. For high flow HPLC applications N2 gas is used to evaporate the solvent or mobile phase and de-solvate the analyte molecules. This is usually unnecessary for capillary columns or nano- applications. After desolvation and charge formation occur, as discussed below, the charged molecules enter a slightly heated transfer capillary tube and pass through two more cones that are used to control the vacuum. Finally, the positively charged ions enter a mass analyzer such as the quadrupole shown in Figure 5.6.
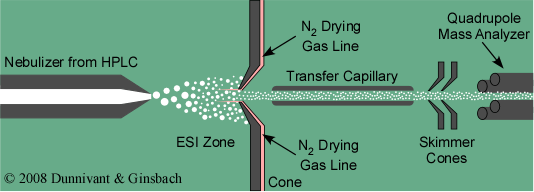
Figure 5.6 Overview of an Electro Spray Ionization (LC-MS) Interface.
The heart of ESI is the desolvation and charge formation shown in Figure 5.7. “Ionization” in ESI is referred to as a soft ionization and is really not ionization but charge formation since no real ionization source is present. Charge formation occurs by evaporating the solvent by passing a dry gas counter current to the movement of droplets. While at the same time the droplets are passed along a charged field (from 2.5 to 4 kV) between the tip of the sample introduction point and the first cone. Charge formation occurs by one of two proposed mechanisms, (1) Ion Evaporation Model where the droplet reaches a certain radius such that the field strength at the surface of the droplet becomes large enough to assist the field desorption of solvated ions and (2) Charged Residue Model where electrospray droplets undergo evaporation and fission cycles, resulting in gas-phase ions that form after the remaining solvent molecules evaporate.
The Charged Residue Model is the most accepted theory and is explained in the following. As the droplets pass from left to right, desolvation occurs in the present of the dry N2 gas. At the same time, the charged field results in the collection of a positive charge on the droplet. As this process continues, from left to right, the droplet shrinks until it reaches a point where the surface tension can no longer sustain the charge accumulation, this point is referred to as the Rayleigh limit. Above the Rayleigh limit, Rayleigh fission (also known as Coulombic explosion) occurs and the droplet is ripped apart forming smaller charged droplets containing the analyte molecules. This process continues until desolvation is complete and the charge is transferred to the ionized and now gaseous analyte molecule. The resulting charged molecules can be singly or multiply charged (refer to Figure 5.7). The positively charged ions enter the mass analyzer. Simple molecules result in a single mass to charge ion while complex molecules result in a Gaussian distribution of mass to charge ions yielding a single molecule molecular mass for identification purposes. As noted above, the ionization process is considered to be a soft ionization, thus, if structural identification is required the parent ion is usually analyzed by tandem MS where it is fragmented into smaller fragments for identification. Nano-spray versions of this process have recently become available.
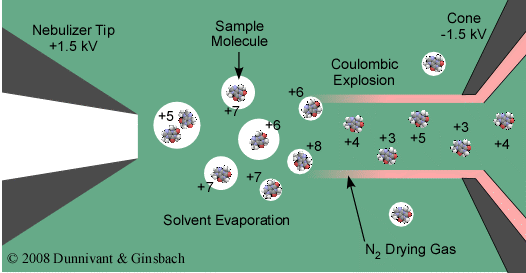
Figure 5.7 Charge Formation in ESI.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008