5.4 The Introduction of Samples from a Capillary Electrophoresis System
Years ago, if you wanted to own a CE-MS system you had to purchase the CE and MS separately and hire the MS manufacturer or vender to interface the two instruments. Recently (~2008) you are now able to purchase off-the-shelf interfaced instruments from chromatography vendors. CE-MS interfaces are designed and operate in much the same way as the HPLC-MS interface, with two exceptions. While HPLC columns can be composed of metal that readily conduct the electrical potential to ionize the analytes, the CE columns are only composed of fused silica. As a result the effluent of the CE column must be coated with a conducting metal sheath. Also, ss you will recall from Chapter 4 on CE, minimal solvent flow results in CE, only from the dragging of solvent by the electrophoretic mobility of the buffer ions. Thus, CE is almost ideal for MS interfaces and is far superior to HPLC interfacing since very little solvent must be removed prior to entry into the MS vacuum system. Other than these two differences, CE-MS operates like HPLC-MS. Solvent droplets, containing analytes, are created at the end of the fused silica column, and are charged by the electrical potential placed between the metal sheath and the metal cone at the entry to the MS system (Figure 5.9). Solvent is evaporated with a drying gas that flows counter current to the movement of the solvent droplets. Charge transfer occurs through Coulombic explosion and the de-solvated and ionized anionic or cations (depending on the potential) are accelerated through the MS interface cone. CE-MS has finally reached a level of maturity and dependability that promises significant advances in many areas of analytical separation and quantification, especially protein studies.
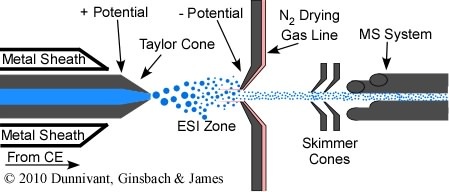
Figure 5.9 A CE-MS Interface.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008