6.10 Fragmentation of Alcohols
The molecular ion of alcohols is usually small and sometimes undetectable especially in tertiary alcohols. In primary and secondary alcohols, the identification of the molecular ion is complicated by the prevalence of a [M – 1] peak caused by the loss of a single hydrogen from the α carbon.
Alcohols also frequently cleave to give resonance stabilized cations due to the breaking of the β bond (Section 6.6). As a result of this cleavage, primary alcohols show a prominent peak at m/z 31 (Figure 6.13).
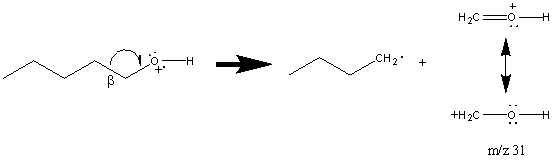
The presence of a m/z 31 peak is not confirmation of a primary alcohol. It is necessary for the peak to be relatively large in comparison to other peaks in the spectrum. This is because secondary alcohols and sometimes even tertiary alcohols can undergo a rearrangement resulting in a peak at m/z 31.
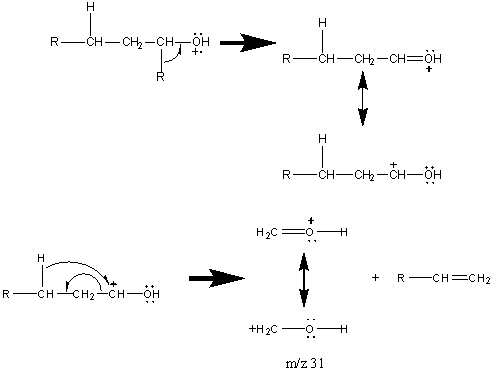
Alcohols also frequently undergo the rearrangement described in Section 6.7 resulting in a [M – 18] peak from the loss of water. This peak is most easily visible in primary alcohols but can be found in secondary and tertiary alcohols as well. Primary alcohols also can lose both water and an alkene.
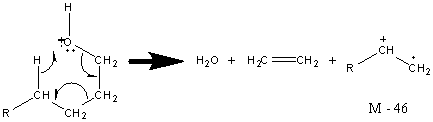
Primary alcohols also produce a [M – 2] peak caused by R-CH=O+ and [M – 3] attributed to R-C≡O+. Alcohols with carbon chains containing methyl groups frequently loose both the methyl group and water at [M – 33].
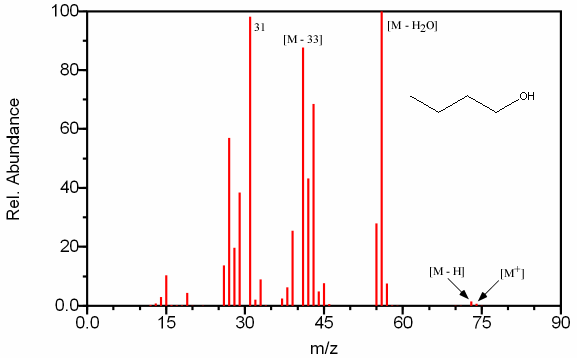
Figure 6.13A (above) can be identified as an alcohol because of the characteristic peak at [M – H2O], [M – 33], and m/z 31. The peak at m/z 31 can attributed to a primary alcohol because it is one of the larger peaks in the spectrum.
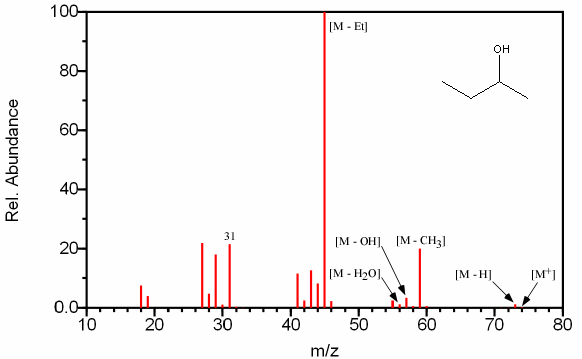
Figure 6.13B (above) must contain an even molecular ion since the major peaks (m/z 59 and 45 are both odd) according to the nitrogen rule. The compound can be identified as an alcohol by the presence of the [M – H], [M – OH], and m/z 31 peaks that are all characteristic of alcohols. The small peak at m/z 31 indicates that this alcohol is not a primary alcohol. The presence of a [M – Et] and [M – CH3] peak indicates that this four carbon alcohol (determined from its molecular mass) is the secondary alcohol 2-butanol.
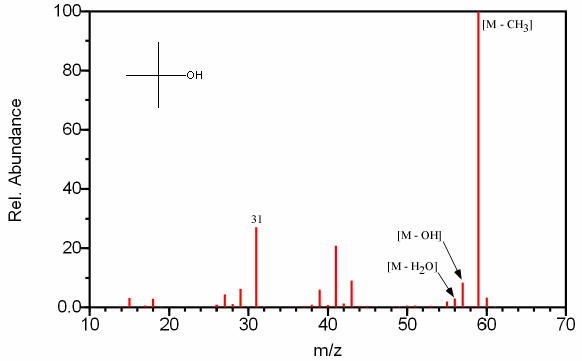
Figure 6.13C (above) lacks a molecular ion since there are illogical fragments from either the m/z 59 or 60 peak. As a result, chemical ionization would need to be utilized to determine the molecular mass is 74. The spectrum recognized as an alcohol by the [M – OH] peak and is easily discernable by the single prevalent peak at [M - CH3].



Figures 6.13A-C. Fragmentation of Three Alcohols. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
Cyclic alcohols fragment similar to straight chain alcohols in that they give a [M – 1] peak from the loss of hydrogen and an [M – 18] peak from the loss of water. They also create a peak at m/z 57 via a complex ring cleavage.
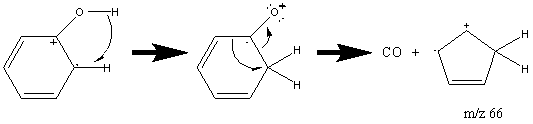
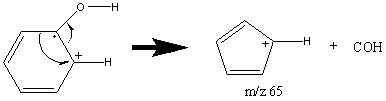
Aromatic alcohols, unlike other alcohols, have a prominent molecular ion peak due to the stability of the aromatic group. Phenols usually give a weaker peak at m/z 77 attributed to a rearrangement and can be identified by two peaks at [M – CO] and [M – COH].
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008