6.11.1 Ketones
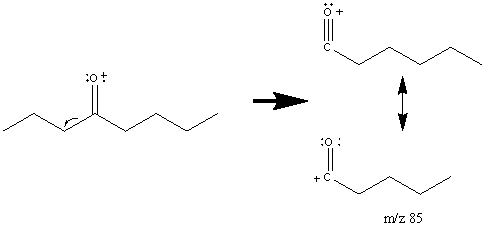
One major fragment of ketones is the creation of the resonance stabilized acylium ion resulting from the cleavage of the α bond. The base peak in the spectrum is usually caused by the removal of the larger alkyl group since it forms a more stable radical illustrated by 4-Octanone (Figure 6.14).
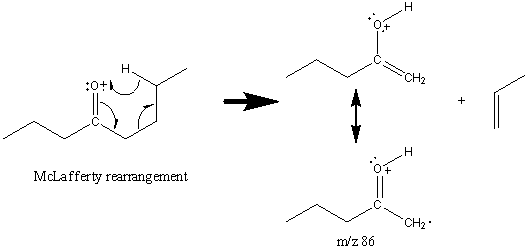
While ketones undergo a single McLafferty rearrangement described above, they also undergo a subsequent McLafferty rearrangement.
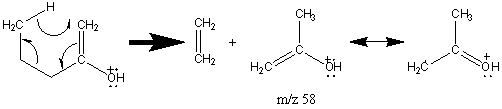
The second rearrangement is mediated by the p system of the alkene group. The ketone functional group is often easily discernable due to the prevalent fragments and rearrangements described above. The configuration of the carbon structure can be difficult to discern. Reduction of the carbonyl group to a methylene group is commonly performed to determine the complete structure of the molecule.
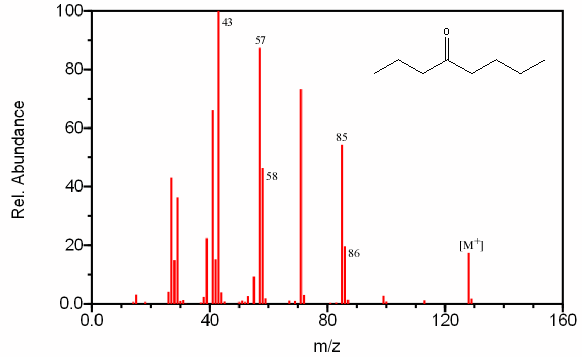
Figure 6.14. Fragmentation of a Ketone. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
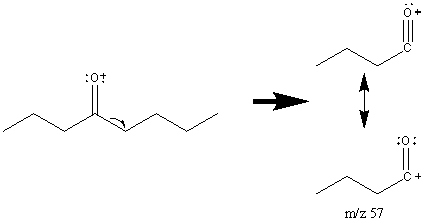
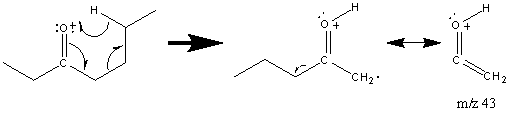
The base peak in Figure 6.14 is the result of a McLafferty rearrangement and an α cleavage.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008