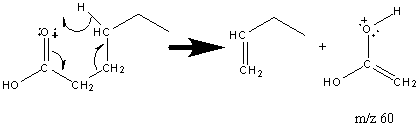
6.12 Fragmentation of Carboxylic Acids
The molecular ion of straight chain carboxylic acids is weak but usually present. The McLafferty rearrangement results in a prominent peak and often accounts for the base peak.

Short chain carboxylic acids give prevalent peaks at [M – OH] and [M – CO2H]. In larger carboxylic acids these peaks are less prevalent. Long chain carboxylic acids are better identified by the fragments at CnH2n-1O2 (Figure 6.17). There is also the presence of the hydrocarbon fragment at CmH2m+1 illustrated in Section 6.9.
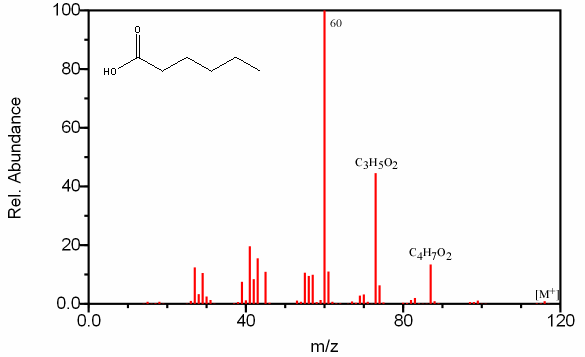
Figure 6.17. Fragmentation of a Carboxylic Acid. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
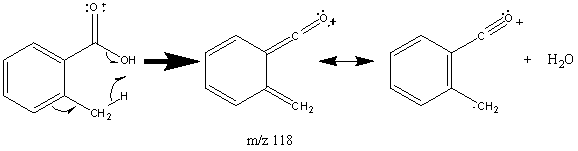
Aromatic acids have a more prominent molecular ion peak but undergo similar fractionation to short chain hydrocarbons. They produce large peaks at [M – OH] and [M – CO2H]. Aromatic acids can also lose water if an ortho group contains an abstractable hydrogen atom.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008