6.15 Fragmentation of Amines
The presence of nitrogen in an amine can be detected by its odd molecular weight and the even fragments that it produces (Section 6.3). Often times the presence of the molecular ion in longer straight chain amines is not detectable. In these cases, chemical ionization techniques are often used in determining the molecular mass in order to determine the presence of nitrogen.
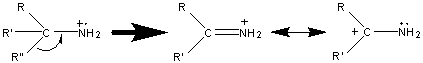
The base peak in most amines results from the cleavage of the β bond. The loss of the largest branch (R”) is preferred because the larger alkyl fragment stabilizes the produced radical.

Like alcohols, if the α carbon is bonded to a hydrogen atom, a [M – H] peak is usually visible. In primary amines with an unbranched α carbon, cleavage of the β bond produces a peak at m/z 30. This peak is not conclusive proof of a primary amine because secondary and tertiary amines undergo a rearrangement similar to that of alcohols.
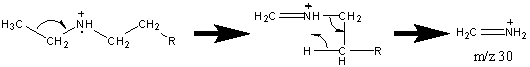
Amines also produce even fragments caused by the cleavage of C – C bonds farther away from the functional group. The fragment containing the nitrogen group usually retains the charge resulting peaks characterized by CnH2n+2N spaced at 14 units. There is also the less prevalent hydrocarbon pattern of CnH2n+1, CnH2n, and CnH2n-1.
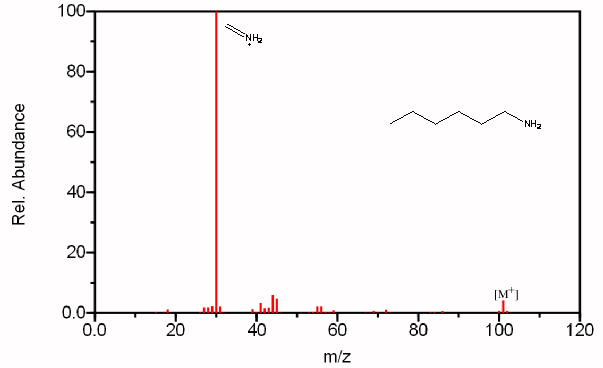
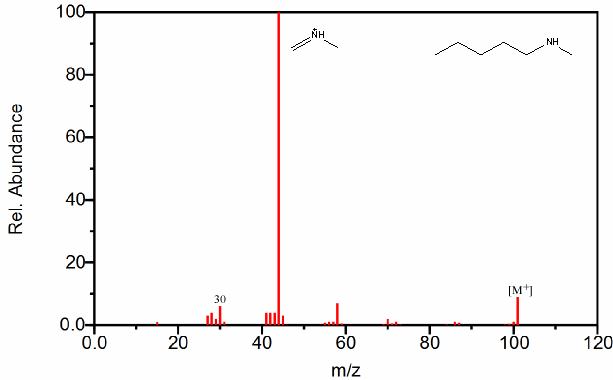
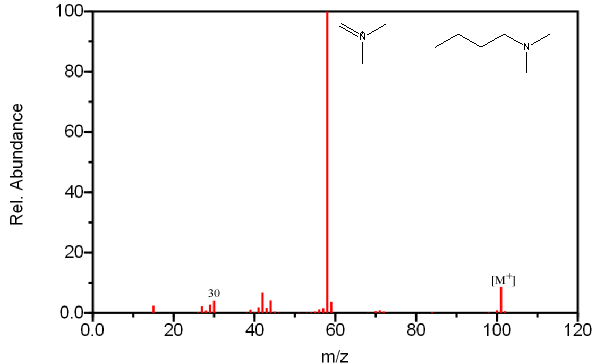
Figure 6.20. Fragmentation of Three Amines - The mass spectrum is easily recognized as an amine due to its odd molecular ion and the presence of even fragments. The base peak in each spectrum, due to β cleavage distinguishes between the primary, secondary, and tertiary amine. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
Cyclic amines produce a discernable molecular ion peak unless the α carbon is substituted. The loss of hydrogen from the α carbon is also a prominent peak. The ring is cleaved when the β bond is broken and subsequent alkene molecules fragment from the remaining ring structure.
The molecular ion of an aromatic amine is expectedly intense. The loss of the hydrogen atom bonded to the nitrogen gives a prominent peak at [M – 1]. Similar to ethers, the loss of HCN from the aniline ion produces peaks at C5H6 and C5H5. Unlike ethers however, the heteroatom not the aromatic group controls the major pathways of fractionation. As a result, the β bond to the amine group is broken.
![]()
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008