6.18 Reviewing General Principals
After surveying common compounds encountered in organic chemistry and their corresponding spectra we are able to make some generalizations about fractionation patterns. When dealing with a compound’s molecular ion;
The molecular ion of ethers, carboxylic acids, aldehydes, and nitrogen containing molecules such as amides and nitriles can be very faint or potentially absent. The molecular ion of alcohols and branched compounds is almost always undetected.
Increasing a compound’s size or the branching of the alkyl portion will decrease the intensity of the molecular ion.
Cyclic structures, elements of unsaturation, and aromatic groups increase the intensity of the molecular ion.
Unknown compounds, in the absence of a reference standard, can often be more difficult but not impossible to discern given the prevalence of multiple fragments. The majority of compounds, however, will abide by the following rules.
Resonance stabilized cations are favored because they help delocalize the positive charge throughout the molecule.
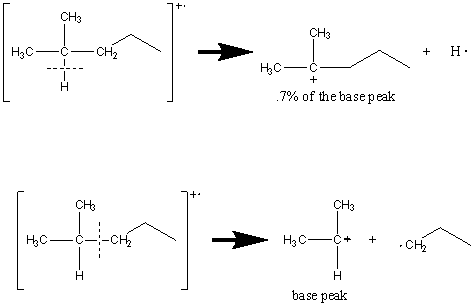
The cleavage of bonds is favored at substituted carbon atoms that produce the most stable cation. As a result, tertiary cations are favored over secondary which are favored over primary which are more stable than CH3+.
The longest chain is eliminated most frequently because the greater number of carbon atoms allows for the delocalization of the radical.
The combination of rules two and three can be good predictors of the prevalence of fragments. Achieving a balance between the stability of the radical and the cation produces the most prevalent peaks. The following example illustrates this point.

The β bond to the heteroatom is frequently broken since the heteroatom’s non bonding electrons allow for resonance forms that stabilize the cation.
Rearrangements account for prominent peaks in the spectrum such as the loss of water from an alcohol or the McLafferty rearrangement.
Besides having a general set of guidelines that govern general fractionation, it is also important to be able to identify patterns that are indicative of particular functional groups. As a result, a condensed table of the commonly observed fragmentation patterns is listed in the table below.
Table 6.3 A Review of Common Fragmentation Patterns.
Functional Group |
Observed Fragments |
M/Z Value |
Straight Chain Alkanes |
CnH2n+1 |
43, 57, 71, … |
[M – CH3] |
[M – 15] |
|
[M – CH2CH3] |
[M – 29] |
|
[M – CH2CH2CH3] |
[M – 43] |
|
Branched Alkanes |
CnH2n |
Various |
Cyclic Alkanes |
[M – H2C=CH2] |
[M – 28] |
Alkenes |
CnH2n-1 |
Various |
CnH2n |
Various |
|
Aromatics |
|
91 |
|
77 |
|
|
56 |
|
Alcohols |
[M – H2O] |
[M – 18] |
[M – (H2O & H2C=CH2)] |
[M – 46] |
|
[M – (CH3 & H2O)] |
[M – 33] |
|
Primary Alcohols |
CH2OH |
31 |
Ketones |
|
43 + R |
|
Various |
|
Aldehydes |
|
44 |
COH |
29 |
|
[M – H2O] |
[M – 18] |
|
[M – H2C=CH2] |
[M – 28] |
|
[M – H2C=CH–OH] |
[M – 44] |
|
Carboxylic Acids |
|
60 |
[M – OH] |
[M – 17] |
|
[M – CO2H] |
[M – 45] |
|
CnH2n-1O2 |
73, 87, … |
|
Ethers |
β cleavage |
various |
|
various |
|
Esters |
|
74, 88, … |
|
various |
|
R’+ |
various |
|
|
various |
|
Amines |
|
various |
CnH2n+2N |
58, 72, … |
|
Primary Amines |
CH2NH2 |
30 |
Amides |
CH2NH2 |
30 |
|
44 |
|
|
59 |
|
|
86 |
|
Nitriles |
[M – H] |
[M – 1] |
|
41 |
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008