6.3 Identifying the Molecular Ion Peak
The molecular ion peak is both an important reference point and is integral in identifying an unknown compound. While it may seem that the molecular ion peak should be the most abundant peak in the spectrum, this is not the case for the majority of compounds. Organic compounds that contain alcohols, nitrogens, carboxylic acids, esters, and high amounts of branching may completely lack a visible molecular ion. In case of an absent molecular ion peak, it is critical that highest molecular weight fragment peak is not mistakenly identified as the molecular ion peak. This mistake would result in misidentification of the analyte of interest. Obtaining a chemical ionization spectrum (CI) can correctly identify the molecular ion (Section 5.2.1.2b).
Even without a CI spectrum of the compound, the mass spectrum provides some information that can assist in ruling out potential masses as the molecular ion. The “nitrogen rule” is one valuable tool for identifying the molecular ion. This rule indicates that if a molecular ion has an odd mass it must have an odd number of nitrogens and that a molecular ion with an even mass must either lack nitrogen atoms or contain an even number of them. Since the majority of organic compounds analyzed with the GC-MS contain either zero or one nitrogen atom, the rule practically states an odd molecular ion is attributed to a single nitrogen and an even molecular ion indicates the sample lacks nitrogen (Figure 6.2). This rule only applies to compounds that contain carbon, hydrogen, nitrogen, oxygen, sulfur, halogens, and a few other less common elements. Since the majority of organic compounds that are analyzed using the GC-MS are made up of these elements, this stipulation is practically ignored.
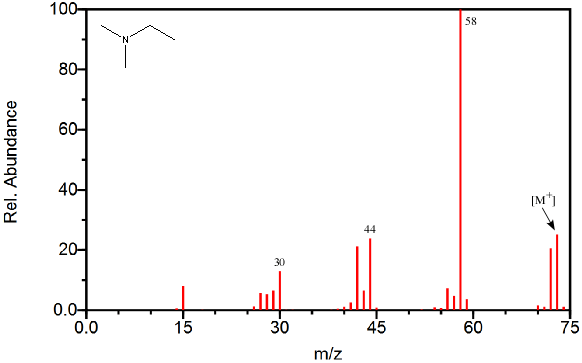
Figure 6.2 The Nitrogen Rule - The mass spectrum of N,N-dimethyl-ethanamine illustrates the presence of an odd molecular ion and even fragments. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
This rule is a result of nitrogen’s unique property. Nitrogen has an even atomic mass but bonds with three other atoms in its most stable form (an even amu plus an odd number of substitutents gives an overall odd molecular mass). Other atoms that have even molecular weights like carbon, oxygen, and sulfur bond with an even number of other atoms (an even amu plus an even number of substitutents gives an overall even mass). Atoms that bond with an odd number of other atoms like hydrogen, chlorine, bromine, and iodine have odd molecular weights (an odd amu plus and odd number of substitutents gives an overall even mass). This rule is invaluable when a chemist knows that a compound lacks nitrogen. This can occur if a sample is prepared from a synthesis whose products and solvents lack nitrogen atoms. In this case, any odd peak cannot be attributed to the molecular ion of the analyzed compound.
Most fragments, excluding rearrangements (Section 6.6), results from breaking a single bond. The nitrogen rule indicates that when a molecule with an even mass produces a fragment by breaking a single bond, the fragment will have an odd mass. When the sample’s mass is odd, fragmentation via a similar pathway will give an even fragment as long as the nitrogen is still contained in the observed fragment. Since this is the observed trend (See Stevenson’s Rule Section 6.6), analyzing the major fragments can help determine if the molecular ion should be even or odd. Practically, if the major fragments are mostly odd, the molecular ion is likely even and contains no nitrogen. If the major fragments are even, the molecular ion is likely odd and contains one nitrogen atom as shown in Figure 6.3.
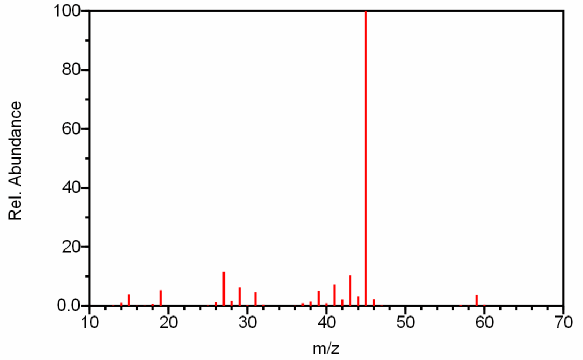
Figure 6.3. The Use of the Nitrogen Rule in Determining the Molecular Ion - Should the faint peak at m/z 60 be attributed to instrumental noise or is it the molecular ion? The presence of the base peak at 45 in combination with our knowledge about the nitrogen rule suggests that the peak at m/z 60 is likely the molecular ion because even molecular ions usually produce odd molecular fragments by breaking single bonds. Given this spectrum is of isopropyl alcohol, our deduction is correct although chemical ionization techniques could verify the molecular mass of the sample. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
Since molecular ions fragment in predictable ways, the presence of certain fragmentation peaks can suggest that a particular peak is the molecular ion. The observed fragments must be able to be attributed to logical losses. The existence of a [M – 15] peak from the loss of CH3, a [M – 18] peak from the loss of H20, or a [M – 31] from the loss of OCH3 are a few examples of these logical fragments.
The opposite is true for fragments that are not logical. These peaks suggest that a particular peak is not the molecular ion. Some illogical fragmentation peaks include peaks that is 3 to 14 mass units away from the peak suggest that the identified peak is likely not the molecular ion peak. The loss of fragments of mass units 1-3 can result from the loss of up to three hydrogen atoms. From 14 to 18, multiple peaks can be explained from the loss of CH3, oxygen, a hydroxide ion, or water. The loss of fragments from the 19-25 range is also unlikely except in the case of fluorinated compounds, which produce [M – 19] (loss of F) and [M – 20] (loss of HF).
The molecular ion can be difficult to identify without chemical ionization because there is no definitive test. While these patterns can greatly assist in identifying the molecular ion, they should not be relied upon for absolute confirmation. Sometimes complex rearrangements can potentially result in the misidentification of the molecular ion. When in doubt, it is good practice to double check with a soft ionization technique such as chemical ionization.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008