6.4 Use of the Molecular Ion
Once the identity of the molecular ion has been determined much can be learned about the compound. One extremely valuable piece of information that can be obtained from a high resolution mass spectrometer is the molecular formula of an unknown analyte. If a molecular ion was identified to have a molecular mass of 80 amu on an instrument with unit resolution, little could be concluded about the molecular formula. For example, some of the many possible molecular formulas include C4H4N2 (80.0375), C5H4O(80.0262), and C6H8 (80.0626). A high resolution instrument measurement of this peak at 80.0372 ± .0005 would indicate that the empirical formula is C4H4N2. Extensive tables and computer programs have been developed to perform this technique on a routine basis.
Once the molecular formula is known, it then becomes possible to determine the degree of unsaturation. This allows the analyst to calculate the number of pi bonds and cyclic structures in the molecule by using the following equation:
Degrees of Unsaturation![]()
where C is the number of carbon atoms, H is the number of hydrogen atoms, X is the number of halogen atoms (F, Cl, Br, and I), and N is the number of nitrogen atoms in the chemical formula. As the equation indicates, the number of oxygen atoms does not affect the degree of unsaturation. This equation indicates that the C4H4N2 compound has four degrees of unsaturation. The molecular mass as well as the corresponding molecular formula simplifies identifyication of the compound.
The molecular ion along with supporting information from IR and NMR spectra allows for complete identification of a compound by combining the information from various techniques. Since IR identifies functional groups, the mass of these groups are first subtracted from the mass of the molecular ion. The remaining mass frequently represents the mass of the carbon and hydrogen contained in a sample. For small molecules, dividing this number by twelve can reveal the number of carbon atoms and a fraction representing the number of hydrogen atoms. It is important to not blindly trust this method. If the molecular ion minus the mass of the functional groups results in 85, dividing by 12 would result in 7 and 1/12 indicating a molecular formula of C7H. Attempting to construct a structure for this molecule will quickly indicate that a more logical molecular formula would be C6H13.
For the unknown analyte illustrated in Figure 6.4, the IR spectrum indicates the compounds’ functional groups. The sharp peak observed at 1710 cm-1 indicates the presence of a carbonyl group. The large round peak centered at 3000 cm-1 suggests that the compound is a carboxylic acid. The large peak slightly above 1600 cm-1 could indicate that the unknown contains a carbon, carbon double bond or even possibly an imine or aromatic functional group.
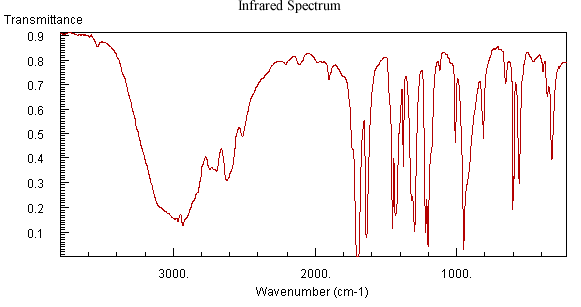
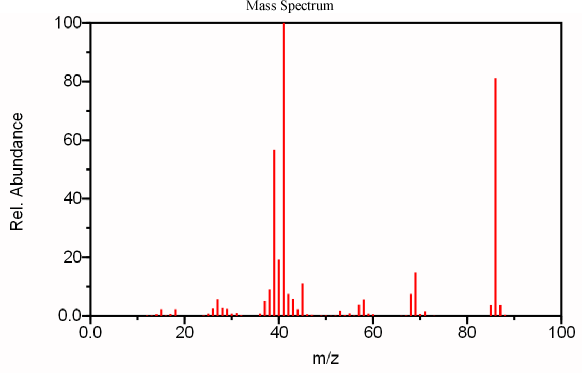
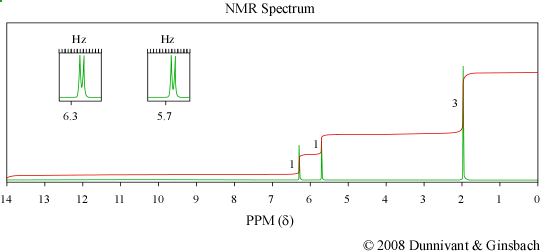
Figure 6.4 IR, Mass Spectrum, and NMR of an Unknown Analyte. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
After obtaining a mass spectrum of the compound, it is necessary to identify the molecular ion. We can identify the peak at 86 to be the molecular ion using the nitrogen rule (discussed above). Because its major fragments are both odd, 69 and 41, it is reasonable that its molecular ion should be even. The peaks at 85 (loss of H) and 71 (loss of CH3) can be explained in a logical fashion further confirming the m/z 86 peak as the molecular ion.
From above, the compound’s mass is 86, thus, we can confirm that the compound is not an imine because it has an even molecular weight indicating that it does not contain an odd number of nitrogen atoms. The molecular weight also indicates that the compound does not contain an aromatic group because molecular mass is too small. Now it becomes possible to deduce information about the carbon backbone of the molecule. By subtracting the mass of the carboxylic acid functional group (COOH) a mass of 41 is obtained. The IR spectrum indicates that the remainder of the molecule is likely only composed of carbon and hydrogen. This allows the analyst to deduce that the rest of the molecule contains 3 more carbon atoms and 5 hydrogen atoms. These two easy measurements establish that this compound’s molecular formula is C3H5COOH. From this molecular formula we can determine that the degree of unsaturation is two. One degree of unsaturation is attributed to the acid functional group while the other is a double bond, since a three-carbon ring is extremely unlikely.
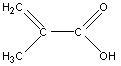
While both the IR and MS are able to determine a great deal about a compound’s identity, NMR is necessary to identify the exact structural formula of this compound. The hydrogen on the carboxylic acid group is absent from the spectrum. The doublet peaks at 6.3 and 5.7 ppm have a coupling constant of approximately 1.4 Hz which indicates that they are geminal protons. The fact that these peaks are doublets indicates that there are only two hydrogen atoms connected to the vinyl group. The presence of a methyl group is indicated by the peak at 2 ppm. As a result the unknown compound is methacrylic acid whose structure is shown below.
Utilizing IR and NMR, in combination with the mass spectrum, made the identification of this compound relatively simple. However, all of these tools are not always readily available. If the analyte of interest is in a complex mixture or there is only a small concentration or quantity (parts per million), both IR and NMR are not effective tools. As a result, it is necessary to be able to identify as much information as possible from the mass spectrum alone. The remainder of this chapter will be devoted to such a task by studying common fragmentation trends in various types of compounds.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008