6.6 Fragmentation
While the molecular ion is one of the most important peaks in the spectrum, it is also important to gain information from the peaks that are a result of fragmentation. The goal of interpreting mass spectra is identifying the structure of the molecular ion by examining pieces (fragments) of the original molecule. The frequency and size of the fragments is dependent on the structure and bond energy of the sample molecule. This property has resulted in the creation of unique and reproducible spectrum for a wide variety of compounds.
Before fragmentation can be discussed, it is necessary to develop a new notation since the cation fragments that will be encountered are not present in other branches of chemistry due to their high reactivity. The presence of the vacuum in the instrument prevents collisions with other molecules allowing these reactive cations to exist. The academic convention for notation is to either represent the charge as a delocalized one (Example A below) or localized it on either a π bond (Example B) or on a heteroatom (Example C).
a) ![]() b)
b) ![]() c)
c) 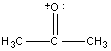
The process that creates these observed fragments is the result of their interaction with the high energy electrons emitted from the tungsten source. This energy first causes an electronic excitation which ionizes a single electron and can also cause the M·+ to enter into an excited electronic configuration. After the molecular ion returns back to the ground electronic state via an emission of a photon, the analyte is still in an excited vibrational and rotational state. Frequently this energy exceeds the activation energy of fragmentation and is released via the breaking of a bond. Experimental data has indicated that this process occurs rapidly in the ionization chamber, before the ion travels through the mass analyzer.
e- + R – R’ ---> [R – R’] ·+ + 2e-
[R – R’] ·+ ---> R· + R’+
[R – R’] ·+ ---> R+ + R’·
The fragmentation of a single bond can produce two cation fragments, one from R+ and the other from R’+. According to Stevenson’s rule, if two fragments are in competition to produce a cation, the fragment with the lowest ionization energy will be formed more often (Figure 6.8).
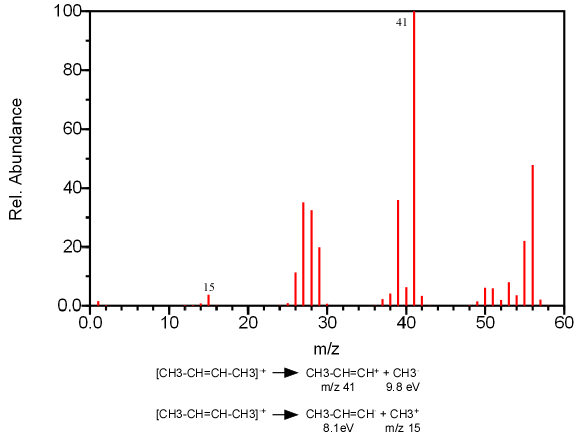
Figure 6.8. An Illustration of Stevenson’s Rule. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
The fragmentation of a bond can proceed through two pathways: either homolytic or heterolytic cleavage. In heterolytic cleavage, a pair of electrons move toward the charged site (illustrated by a double headed arrow) producing a cation and a radical.
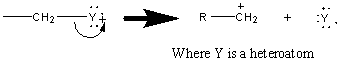
The fragmentation produced by a homolytic cleavage results from the movement of two single electrons, splitting a bonding pair.
![]()
For simplicity, usually only one arrow is drawn to illustrate the movement of electrons.
![]()
These fragmentation patterns are usually the result of a functional group contained in the compound. As a result, the bonds that typically break are either located one, or two carbon atoms away from the functional group. These atoms are referred to as the α and β carbons.
![]()
The bond between the functional group Y and the α carbon is called the α bond and the bond between the α and β carbons is the β bond.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008