6.7 Rearrangements
Some fragments are the result of the cleavage of multiple bonds. The removal of water from an alcohol is one example. The nitrogen rule (Figure 6.3) is helpful in identifying peaks that are produced via a rearrangement. If a molecular ion has an even molecular weight then generally peaks of even molecular weight were created from a rearrangement. If a molecule has an odd molecular weight, then its rearrangements peaks will also be odd.
One rearrangement is the loss of water from a primary alcohol. The mechanism is illustrated with butanol.
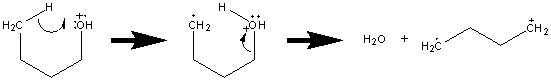
Unlike homolytic or heterolytic cleavage, rearrangements must go through some intermediate transition state. This transition state is frequently the result of some specific orientation referred to as a tight complex. While this tight complex hinders the rate of the reaction, rearrangements are favored because the new bonds help stabilize the products. Other rearrangements such as the McLafferty rearrangement will be explored in greater detail in the following sections.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008