6.9.3 Fragmentation of Cyclic Alkanes
The ring structure of cyclic alkanes increases the intensity of the molecular ion. It’s stability also increases the likelihood that side chains will fragment at the bond α to the ring. The fragmentation of the cyclic structure is usually caused by the loss of more than two carbon atoms. The loss of a methyl radical occurs less frequently because of the instability of the methyl radical in comparison to the neutral ethylene molecule at [M – 28] or an ethyl radical at [M – 29].
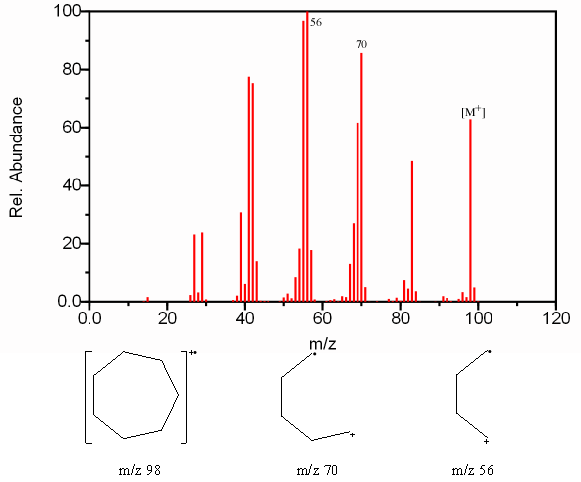
Figure 6.11. Fragmentation of a Cyclic Alkane. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008