6.9.5 Fragmentation of Aromatics
The presence of an aromatic ring in a compound results in a prominent molecular ion. A common peak at [M – 1] results from the loss of a hydrogen atom from the benzene ring. Alkyl substituted benzene rings result in a prominent peak at m/z 91 (Figure 2.12). In most cases, the peak at m/z 91 is the result of a tropylium ion caused by the following rearrangement.
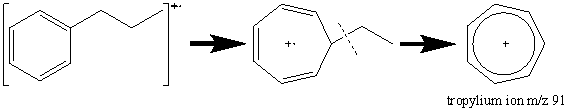
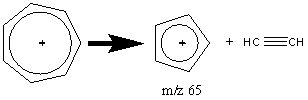
The peak observed in most aromatic compounds at m/z 65 results from the elimination of an acetylene molecule from the tropylium ion.
Benzene rings with highly branched substituted groups produce fragments larger than m/z 91 by intervals of 14 units. The largest of these peaks will result in a highly substituted cation and a large radical, like a simpler branched alkane. The fragment at m/z 105 in Figure 2.12 is relatively small since it produces a primary carbocation and an unstable methyl radical. Substituted benzene rings also first undergo α cleavage followed by hydrogen rearrangement producing a grouping of peaks at m/z 77 from C6H5+, m/z 78 from C6H6+, and m/z 79 from C6H7+.
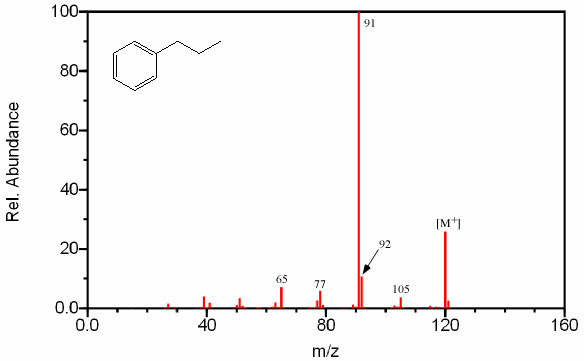
Figure 6.12. Fragmentation of an Aromatic.Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
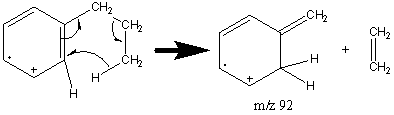
Side chains with more than two carbon atoms create a peak at m/z 92 (Figure 6.12). Unbranched side chains result in a more prevalent peak at m/z 92 than do branched groups.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008