7.3.2 Advantages of GC over MS; cis- versus trans-
The experiment in Section 3.3.1 illustrated the power of MS in identifying analytes when they could not be separated by GC. This experiment will do the reverse, use GC to identify analytes that give the same spectra with MS. This is important with cis- and trans- isomers. Cis- and trans- isomers can have significantly different physical parameters due to the rotation of functional groups around a double bond. For example, cis-stilbene has a boiling point of 82-84 C, while rotation of one benzene ring around the double bond to form trans stilbene yields a boiling point of 305-307 C. These can easily be separated by chromatography but ~all cis- and trans- isomers yield the same fragmentation pattern in MS.
cis-stilbene trans-stilbene
This experiment will use GC to separate and identify cis- and trans- heptene. Look up the boiling points to estimate the relative retention order.
Experimental Procedures
Chemicals and Supplies:
-A 50 ppm solution of cis- and trans- in heptene
GC-MS Settings:
-Capillary Column: DB-5: Poly(phenylmethyldimethyl) siloxane (5 % phenyl)
30 m x 0.25 mm; 0.25 mm phase coating
-Injection Volume: 1.00 mL
-Splitless Injection for: 0.50 min.
-Split Flow Rate: 50 mL/min.
-Column Flow: 1.2 mL/min.
-Linear Velocity: 40 cm/s
-Injector Temperature: 250 C
-Detector Temperature: 230 C
-Quadrupole Temperature: 250 C
-Oven Program: 80 C for two minutes, 5 C/min to 210 C, hold for 10 minutes
Procedures:
Analyze the standard solutions on a GC-MS using the instrumental conditions given above.
RESULTS:
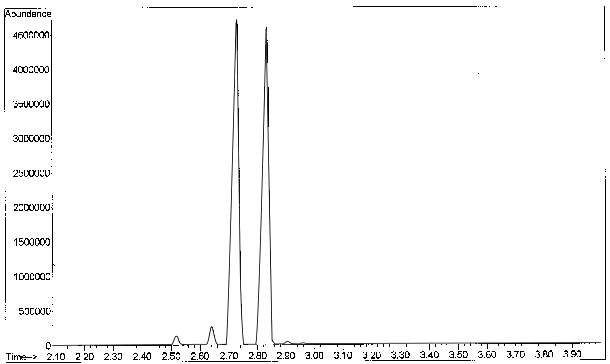
Figure 7-11. Total Ion Counts for the Analysis of cis- and trans- Heptene.
Note the dependence of the results, retention times, on boiling points. DB-1 and DB-5 columns separate exclusively based on boiling points.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008