1.2 The Interaction of Electromagnetic Radiation with Sample Molecules
Various types of interactions (both absorption and emission spectrometry) are utilized to measure the concentration of metal analytes. Despite these different techniques, they both rely upon the excitation of valence electrons. This is caused by the absorption of energy from a collision or energy from a photon. The absorption of a photon by a chemical species occurs when electromagnetic energy is transferred to the chemical species. In all cases these transitions are wavelength specific, meaning that the energy of the photon must very closely match the energy of the electronic transition. For a given molecular species the energy is the sum of the electronic, vibrational, and rotational energies. However, an atomic species cannot undergo vibrational or rotational excitations since there are no chemical bonds, thus only electronic energy levels are shown in the figure below. After a transition occurs, a number of relaxations can be measured for a wide variety of analytical purposes. For the purposes of this text, only electronic transitions are discussed in determining the presence and concentration of a metal analyte. Figure 1-1 below summaries the possible ways an energy excitation could occur in an atomic (or molecular) species. Recall that the difference in two adjacent energy levels is not constant but is instead a function of the type of transition. For example, moving up an electronic energy level requires more energy than moving up a single vibration or rotational level. If a photon is not absorbed by a chemical species, it is transmitted through a sample and is left unaltered by the sample media.
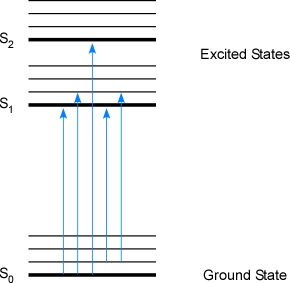
Figure 1-1. A Jablonski Diagram Showing Electronic Energy Transfer Levels for Singlet States in Atomic Species. (S represents the electronic energy level of a singlet electron; the smaller lines above each electronic energy lines represent possible rotational levels)
The energy of electromagnetic radiation utilized in the analytical technique must match the energy of the transition of interest. It is important to recall the energy/frequency/wavelength associated with different types of radiation described in Figure 1-2. Table 1.2 summarizes the usual energy transitions that result from each type of radiation. The analytical techniques discussed in this text usually only utilize UV or visible radiation, since these photons have sufficient energy to excite valence electrons in atomic species.
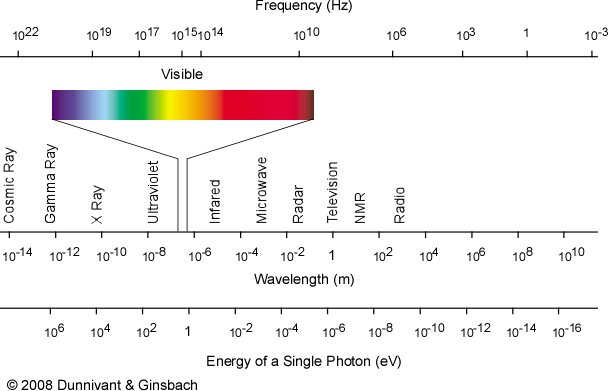
Figure 1-2 The Electromagnetic Spectrum.
Table 1.2 Typical Atomic and Molecular Energy Transitions Associated with Each Type of Photon and Photon Wavelength.
| x-rays | Angstroms to 0.1 nm | Can promote or remove inner (core) electrons |
| Vacuum UV | 10-190 nm | Can break molecular bonds. Results in the removal or promotion of electrons to excited states |
| UV | 190-300 nm | Can break molecular bonds. Results in the removal or promotion of electrons to excited states |
| Visible | 350 to 800 nm | Results in the removal or promotion of electrons to excited states |
| IR | 0.8-300 μm | Increases the amplitude of vibrations |
| Microwaves | ~1-4 mm | Increases the rate of molecular rotation |
The other factor that determines the wavelength of a given absorption is the type of atomic species. For example, Figure 1-3 shows the possible electronic transitions of a 3s electron of atomic sodium. The most likely transitions are indicated by the thicker yellow lines at 589.0 and 589.6 nm. These spectral lines are utilized for analytical measurements and are so intense that they can be observed by the human eye. Yellow light is emitted when sodium-containing compounds are placed in a common flame. These sodium atoms are excited by the flame and subsequently relax by releasing a photon which is then observed by our eye. These types of electronic transitions and energy relations should be familiar to the reader from basic first-year general chemistry principles where fundamental equations such as
![]()
were used in combination with Bohr’s equation (for hydrogen)
![]()
where E is energy, c is the speed of light in a vacuum, λ is a photon’s wavelength, υ is a photon’s frequency, h is Planck’s constant, R is Rydberg’s constant, ni is the initial electronic energy level, and nf is the final electronic energy level. Thus, electronic transitions such as n1 to n2, can be related to energy, frequency, and wavelength by the equations given above.
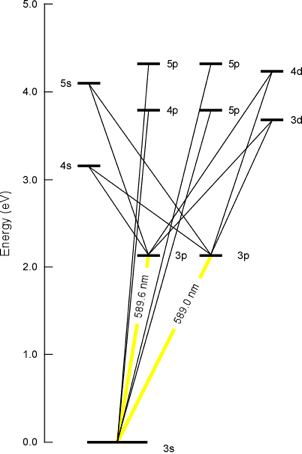
Figure 1-3. Possible Electronic Transitions for a 3s Electron in a Sodium Atom. (Note the two different 3p energy levels, one with a higher energy for j=3/2 and a lower energy with j=1/2).
The possible transitions shown in Figure 1-3 are specific to a particular element and such a diagram can be constructed for every metal. When energy (or a photon) corresponding to a specific transition is absorbed, the electronic state is changed (i.e. from 3s to 3p). Some metals have only a few likely electronic transitions while others will have more. Usually, the most prominent wavelength produced by this transition is used in instrumental absorption and emission measurements. Atomic absorption spectrometry (AAS), atomic emission spectrometry (AES) and inductively coupled plasma-atomic emission spectroscopy (ICP-AES) are only concerned with the absorption of a photon by a ground state atomic species, and the emission of photon from a singlet electronic level.
This absorption of a photon is one potential way that FAAS will produce signal data. Sometimes, the absorbance of a sample is converted to transmission by the following equation:
Absorbance = - log(Transmission)
Transmission = I / Io
where I is the intensity of light passing through a sample and Io is the intensity of light passing through a blank sample (reference sample cell).
In addition to absorption and transmission of photons, scattering of UV/visible radiation is another common interaction of electromagnetic radiation with matter. The scattering of radiation by large molecules or small particles can be a very useful phenomenon, and is the basis for other analytical techniques such as Raman spectroscopy or photocoupled spectroscopy, respectively. However, for the instruments discussed here, photon scattering is a detriment to analytical measurements since it reduces the intensity of radiation that interacts with a sample. Scattering is caused when the electric field of a photon interacts nonresonantly with the energy level structure of an atom or molecule. Scattering is a problem when stray photons occasionally reach the detector creating a false analyte signal. In optical spectrometry, scattered light is prevented from reaching the detector by non-reflective black surfaces inside the instrument, and by enclosing the detector in a separate compartment.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008