2.2 Components of a Flame Atomic Absorption/Emission Spectrometer System
2.2.5 Burner Head
The burner head in FAAS and FAES systems is where all of the chemical reactions take place. The burner head, as shown in Animation 2.2, consists of an inlet tube; fuel and air inlets; a nebulizer; mixing cell; and the flame (the reaction and sample cell). Aqueous samples move through the inlet tube into the nebulizer which atomizes the liquid into small droplets. The mixture of sample droplets, oxidant, and fuel is homogenized by the mixing fins in the mixing cell before this mixture is atomized in the flame. The sample liquid is drawn into the nebulizer by a phenomenon known as the Bernoulli effect where a compressible fluid (the fuel and oxidant gases) is passed through a constriction in a pipe.
The Bernoulli principle is the pressure differential created when gases flow through a constriction. The gaseous flow, where the velocity is below the speed of sound, creates streamlines along the path of flow (represented by the horizontal lines in Figure 2-3). A streamline is an imaginary line that describes the path of a gaseous molecule through a system operating under laminar flow (a flow system with little random motion or mixing). The Bernoulli principle states that the sum of the mechanical energies along a streamline is the same at all points on that streamline. This requires that the sum of kinetic energy and potential energy remain constant along the streamline. If the gas is flowing out of one reservoir (the reservoir with a larger radius) into a constricted reservoir, the sum of all forms of energy along the way is the same on all streamlines. The total energy at any point can be described by the following equation
![]()
where γ is the ratio of the specific heats of the fluid, p is the pressure at a point, ρ is the density at the point, ν is the speed of the fluid at the point, g is the acceleration due to gravity, and h is the height of the point above a reference plane. Since the total energy of the system must be conserved, the total energy must equal a constant for the system.
Consider a gas molecule moving from left to right along one of the horizontal streamlines in Figure 2-3. While all of the variables in the total energy equation change, it is necessary to only focus on the pressure and the velocity at a particular point. This is valid because the pressure and the velocity at a single point are the dominant contributors to the overall total energy. When this molecule moves from left to right it encounters less pressure and subsequently h becomes smaller. In order for energy to be conserved, the velocity (ν) must become larger. The converse is true when the particle moves farther right into an area of higher pressure. In this instance, the velocity must become smaller so the total energy of the molecule never changed over the entire system. As a result of Bernoulli’s equation, the highest speed occurs at the lowest pressure, and the lowest speed occurs at the highest pressure.
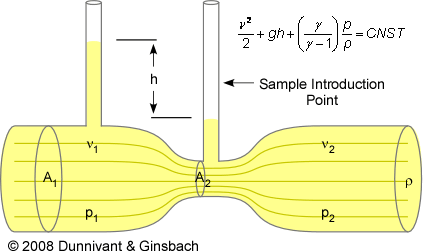
Figure 2-3 Diagram Explaining the Bernoulli Principle (A is cross sectional area, v is the fluid velocity, p is pressure, ρ is fluid density, and h is head pressure (or difference in pressure).
The Bernoulli discussion (and Figure 2-3) is only illustrative of the general concept of pressure and flow balances. The design of the inlet chamber in AAS units is slightly different, but the principles of the Bernoulli equation cause the sample to enter into the mixing chamber in AAS and AES units. For the situations occurring in FAAS and FAES, if a fluid reservoir (the aqueous sample inlet tube) is connected to the low-pressure region of the fuel and oxidant gas inlet constriction, the lower pressure present in the constriction will draw fluid into the system (nebulizer chamber). A compression valve located on the sample inlet pipe is used to regulate the flow. As a result, the Bernoulli effect causes the liquid to freely move into the AAS without the use of a pump.
Next, the sample enters into the nebulizer, a mixing chamber where the sample is broken into an aerosol mist. The droplet size of this aerosol formed in the nebulizer is of importance since this directly affects how much analyte reaches the flame. Droplets with diameters greater than 20 μm are trapped in the spray chamber by attaching to surfaces and flow to the waste container. Only about 10% of the water that enters the nebulizer reaches the flame. The empirically determined governing equation for the determination of droplet size is
![]()
where do is the droplet size which is a function of viscosity (η), density (ρ), and surface tension (γ) of the sample solution, the flow rate of the nebulizer gas (Qgas) and the aspirated solution (Qliq), and the velocity of the nebulizing gas (ν).
After the sample is pulled into the nebulizer and turned into an aerosol mist, it is mixed with the fuel and oxidant by two mixing fins. Common fuels used in FAAS and FAES units are acetylene (for hotter flames) and hydrogen (for cooler flames). Oxygen in the air or nitrous oxide is used as the oxidant to regulate the temperature of the flame. Different elements require different flame conditions, including the choice of fuel and oxidant and the ratio of the fuel to oxidant mixtures. Hydrogen-air flames produce temperatures of about 2000 °C, while acetylene-air flames yield temperatures of approximately 2300 °C and acetylene-nitrous oxide yield temperatures of 2900 °C. Within these fuel types, fuel-rich mixtures yield cooler flames and oxidant-rich mixtures yield hotter temperatures. Temperatures are optimized for a particular analyte since different metal elements are excited or atomized under different conditions. In addition, some metals readily form oxides in an oxygen rich atmosphere, a reducing (fuel rich) environment is necessary to produce atomic instead of molecular species (such as oxides). Other elements are stable in the atomic state under any fuel/oxidant mixture. After this specific mixture of fuel and oxidant are mixed together with the sample, they exit the burner head and pass into the flame.
Several processes and reactions occur rapidly when the sample molecules enter into the flame. First, the water is evaporated and removed from the metal complex. Next, the heat of the flame degrades organic and dehydrated inorganic complexes into gaseous atomic states (ground electronic states) that are then excited by the thermal energy in the flame. In the lower portion of the flame, absorption of photons occurs by the electronic ground state gaseous atoms. As the analytes rise into cooler regions of the flame, the excited atoms relax and emit a photon for emission spectrometry. Finally the fumes and metals from the flame are removed from the laboratory by a fume hood exhaust system.
Please review the animation of the sample introduction system and the burner head in Animation 2.2.
Animation 2.2 Illustration of an Aqueous Sample Introduction into a Burner Head.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008