6.9.1 Fragmentation of Straight Chain Alkanes
Straight chain alkanes always produce a molecular ion, even in long chain compounds where the molecular ion is usually faint. The base peak in the spectra is usually the peak at m/z 57 corresponding to the C4H9 carbocation surrounded by other smaller peaks due to the rearrangement of hydrogen atoms. These groups are separated by 14 mass units resulting from the loss of another CH2 group. The largest peak in each cluster is caused by the loss of (CH2)nCH3 resulting in a fragment of molecular formula CmH2m+1. The peak heights corresponding to subsequent fragments after the C4 peak decrease in an exponential fashion to a minimum at [M –C2H5]. The [M – CH3] peak is weak in smaller compounds and absent in long chain compounds due to the relative instability of the methyl radical. The molecular ion is the unique peak in straight chain alkanes longer than eight carbon atoms.
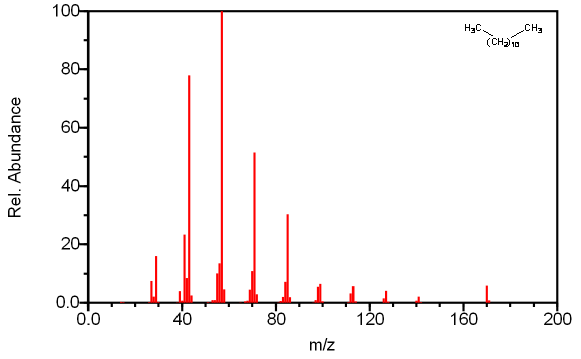
Figure 6.9 Fragmentation of a straight Chain Alkane. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
The example illustrated in Figure 6.9 illustrates these common trends discussed above. The prominent peaks at CmH2m+1 combined with the decaying intensity of these peaks indicates this compound is an alkane. The base peak at m/z 57 caused by C4H9 is further confirmation that there is a lack of other functional groups causing other prominent fragments. The molecular ion at m/z 170 indicates that this compound is dodecane.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008