3.3 Components of an Inductively Coupled Plasma—Atomic Emission Spectrometry System (ICP-AES)
3.3.1 Overview
An ICP-AES system can be divided up into two basic parts; the inductively coupled plasma source and the atomic emission spectrometry detector. Figure 3-1 shows the common components of an ICP-AES system from the late 1980s to the 1990s. The inductively coupled plasma source has mostly been unchanged since its invention with the exception of innovation in monochromator type, which enables greater suppression of interference phenomena. Modifications of this common system will be explained in the following sections.
Sample solutions include digested soil or other solid material or natural water. Typically the sample solution is acidified up to 2-3% in HNO3 to prevent adsorption of metals onto polypropylene sample bottle or onto instrument tubing or glassware prior to introduction into the plasma. In Figure 3-1, the sample is introduced to the nebulizer chamber via a peristaltic pump and tygon tubing attached to an automatic sampler. A peristaltic pump operates by sequentially compressing flexible tubing with evenly spaced and rotating rollers that pull/push the liquid through the system. The rate of sample introduction into the plasma changes as the rotation rate of the peristaltic rollers increases or decreases. Flow of sample and Ar gas through the small aperture of the nebulizer creates very small droplets that form a mist of μm-sized particles in the nebulizer chamber. Larger sample droplets collect on the chamber walls and are removed through a drain, while smaller particles travel with the Ar flow and enter the torch. Evaporation, atomization, and excitations/ionizations occur in the plasma at temperatures reaching 10 000 K. Ar not related to the sample is also excited and ionized because this gas both carries the sample aerosol and confines the location of the plasma to prevent damage to the rest of the instrument. As the excited/ionized atoms leave the hot portion of the plasma, excited valence electrons relax and emit a photon characteristic of the electron transition. This photon is specific to the element but does not yield any information about the isotopic state of the element, unlike in mass spectrometry (Chapter 4). Visible and UV radiation emitted from the sample constituents enters the monochromator through a small slit where the wavelengths are separated by grating(s) and/or prism(s) before being captured and measured by a wide variety of detectors.
Because spectral interferences may still occur, the choice and configuration of the monochromators in the instrument is important and has been the target of innovation. In Figure 3-1, the most common form of a monochromator (a Rowland circle) and detector (photomultiplier; PMT) is shown: The Rowland system utilizes a concave Echellette-style grating monochromator to separate the various emission lines and simultaneously focus individual wavelengths on to a series of slits, with each slit aligned to allow a specific wavelength of radiation to pass to a detector. The standard detector, a photomultiplier tube (PMT), was discussed in Section 2.2.9. Some systems use multiple PMTs at fixed locations to monitor each wavelength simultaneously (Figure 3-1) whereas other systems use a single PMT and move it to different locations to detect each wavelength. Data from these detectors are processed by a computer because multiple wavelengths are measured in an ICP-AES system at the same time.
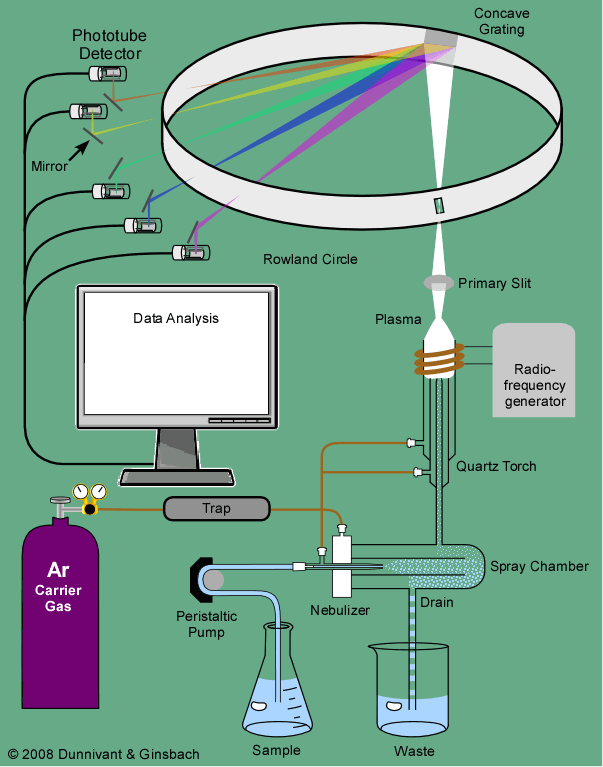
Figure 3-1. Overview of a Basic Inductively Coupled Plasma—Atomic Emission Spectrometry (ICP-AES) from the 1990s.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008