4.2.1: Electro-Osmotic Flow (EOF)
Also called electroendosmotic flow, electro-osmotic flow is the movement of liquid in a porous material (such as a capillary tube) caused by a difference in potential across the material.
When a charged particle is put in contact with a liquid in a capillary tube, a double-layer--or electrical double layer (EDL)--forms at the wall of the capillary (see Figure 4.2); this occurs at the interface of the glass capillary wall and the bulk solution. The first layer is surface charge, and can be positive or negative depending on the material. As capillaries are generally borosilicate glass, the numerous silanol (SiOH) groups cause the charge of the first layer to be negative. This layer is also sometimes called the Stern layer or Helmholtz layer. The second layer is made up of ionic particles in solution that are electronically attracted to the charge of the capillary surface. As the particles in this layer are not fixed, but move as a result of electrical and thermal energy, it is called the diffuse layer.
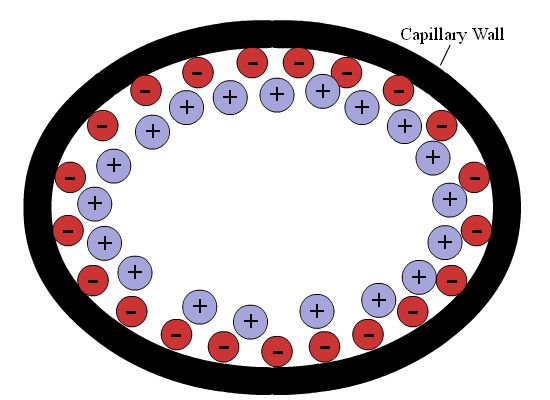
Figure 4.2: Capillary double-layer
The diffuse layer has a net charge, whereas the bulk, uncoordinated solution generally does not. When an electrical potential is placed across the capillary tube, the diffuse layer is pulled to one side. As the diffuse layer progresses to one side of the capillary tube, it drags the bulk solution along with it, creating a flow (specifically, the electro-osmotic flow) of the solution through the cathode. Because the species in the solution are charged, they separate in solution as discussed above; for most applications*, positively-charged analytes move toward the anode given normal (negatively charged capillary) conditions.
The electro-osmotic flow (EOF) can be described in terms of velocity or mobility. The mobility can be calculated from Equation 4.6, where: ε (unitless) is the dielectric constant, a measure of a material's ability to respond to a dialectric's ability to store charge; ζ (V) is the zeta potential, discussed later; and η (Pa s = kg/s m) is the solution viscosity.
![]() Eqn 4.6
Eqn 4.6
Since the mobility is the ability to respond to electric potential, the velocity is the mobility multiplied by the electric potential:
![]() Eqn 4.7
Eqn 4.7
The zeta potential (ζ) is the potential due to the double layer on the capillary wall, and is directly correlated with the charge on the capillary. Because the charge on the capillary varies as a function of pH, the zeta potential also varies with pH, meaning the mobility and velocity of the EOF are highly pH dependent. At high pH, the silanol groups are deprotenated, meaning the net charge of the capillary wall is greater. Thus, the EOF is significantly greater at high pH than at low pH; in fact, the EOF can sometimes vary more than an order of magnitude between a pH of 2 and a pH of 12.
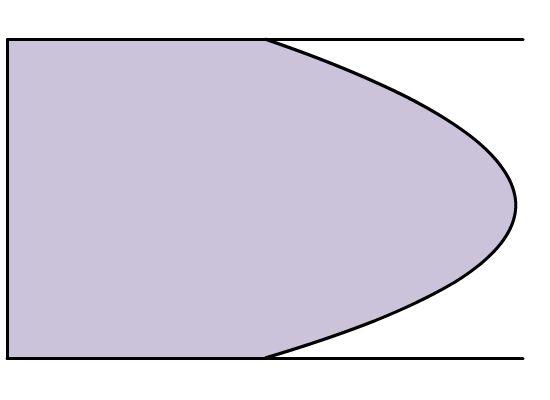
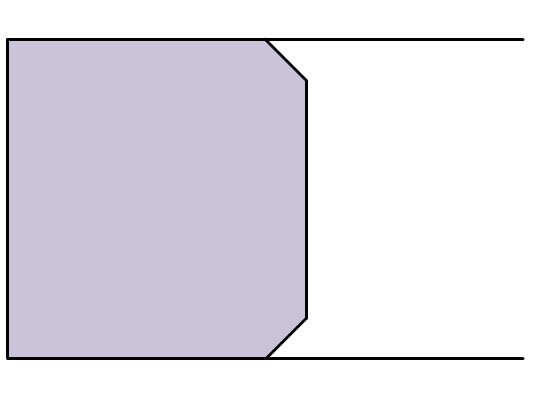
Figure 4.3: Pump induced flow (left) vs. electro-osmotic induced (right) flow
A marked difference between electro-osmotic flow and flow induced by a pump is the cross section of the flow. Because a pump applies a force across the entire cross sectional area of the liquid in a capillary or tube, the friction between the wall and the liquid will cause the liquid at the center of the cross section to move faster than the liquid closer to the wall. Thus, the liquid velocity gradient flows parabolically down through the capillary. However, if a liquid is moved by electro-osmotic flow, the bulk flow is caused primarily by the acceleration of the cations near the capillary wall, and the force on the liquid is uniformly distributed. This results in a flat flow profile. As seen in (Figure 4.3), the flat flow profile drops off sharply at the capillary wall; this is caused by friction against the direction of flow, but to a much lower extent than observed in laminar flow caused by pumps. An even (or flat) flow results in compact peaks whereas a parabolic laminar flow results in a broad peak because the analyte is spread over a larger area. "Tighter" peaks are easier to integrate and facilitate the isolation of peaks that elute at similar times. Table 4.1 summarizes the variables that control electro-osmotic flow.
Table 4.1: EOF variables
| Variable | Effect on EOF | Possible Problems |
| pH | directly proportional | an alter structure and charge of analyte |
| Ionic Strength | inversely proportional | sample heating and adsorption |
| Temperature | inversely proportional | changes in viscosity |
| Electric Field | directly proportional | can cause heating |
| Organic Modifiers | usually inversely proportional | can alter selectivity of instrument |
| Hydrophilic Polymer | usually inversely proportional | dependent on polymer |
| Covalent Coating | dependent on coating coating | often unstable |
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008