6.11.3 Fragmentation of Aromatic Ketones
Aromatic ketones create fragments via almost identical pathways as aliphatic ketones. One prominent peak, and usually the base peak, is the result of the cleavage of the less stable alkyl fragment resulting in the ArC≡O fragment located at m/z 105. The alpha cleavage resulting in a benzyl radical is infrequent given the stability of the competing reaction (Figure 6.15).
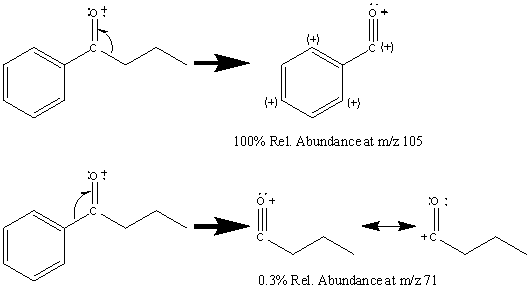
The cleavage of the bond α to the aromatic group results in a peak at m/z 77.

Further fragmentation results in a peak at m/z 55 after the loss of HC≡CH. Some aromatic ketones undergo the typical McLafferty rearrangement if the other alkyl component contains an abstractable hydrogen atom.
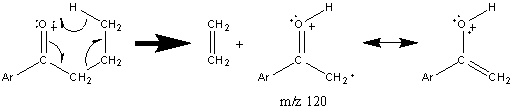
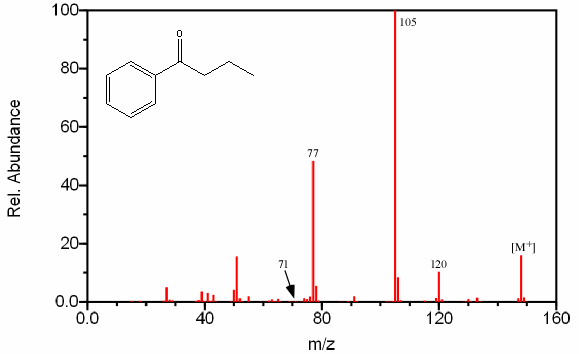
Figure 6.15. Fragmentation of a Cyclic Ketone. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008