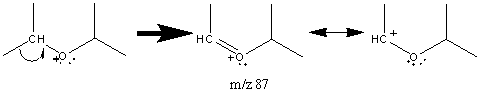
6.13 Fragmentation of Ethers
The molecular ion peak is usually weak in ethers. The oxygen atom mediates the major fragment and creates a β cleavage that results in a resonance stabilized cation. This peak is prominent and sometimes is the base peak.
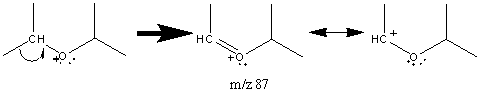
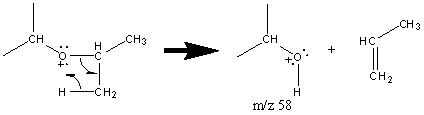
The fragment can also undergo a subsequent rearrangement which typically creates the base peak when the α carbon is substituted.
Ethers also produce prominent alkyl fragments when the C–O bond (α bond) is broken and the fragment containing oxygen is a radical.
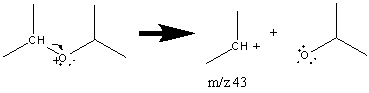
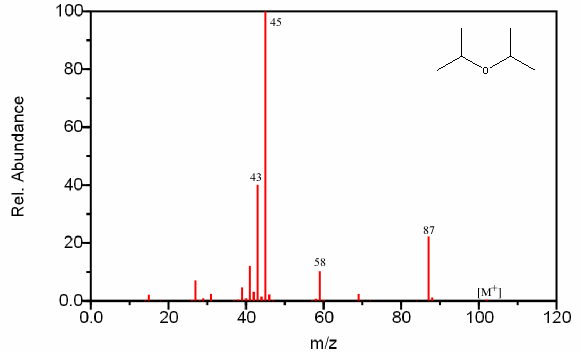
Figure 6.18. Fragmentation of an Ether. Spectra from the NIST/EPA/NIH Mass Spectral Library. Reprinted with permission from NIST.
The base peak in Figure 6.18 is the result of both a β cleavage and the above rearrangement.
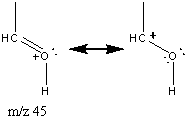
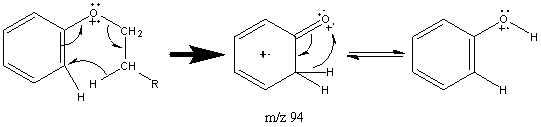
Aromatic ethers have a slightly different pattern of fragmentation. They produce prominent molecular ions due to the stability of the benzene ring. The major fractionation occurs at the β bond to the aromatic ring. This fragment can decompose further with the loss of CO.
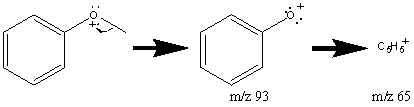
Aromatic ethers also cleave at the bond α to the ring to create a peak at m/z 77 and 78 due to hydrogen migration.
When the alkyl portion of the sample is larger than two carbons, the β is accompanied by hydrogen migration caused by the presence of the aromatic group. This cleavage results in a peak located at m/z 94.
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008