6.16 Fractionation of Amides
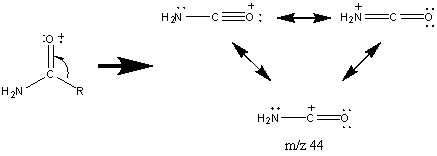
The molecular ion of most straight chain amides is usually discernable which allows the nitrogen atom to be identified. The fractionation pattern is dependent on the length of the alkyl chain and the degree of substitution of the nitrogen group. The base peak in primary amides that lack an abstractable hydrogen atom for the McLafferty rearrangement occurs at m/z 44.

In the other amides including secondary and tertiary, the base peak is created by the McLafferty rearrangement. Primary amides also produce a peak at m/z 86 as a result of the following cleavage.
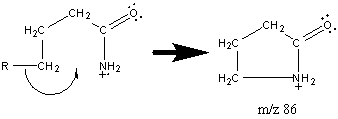
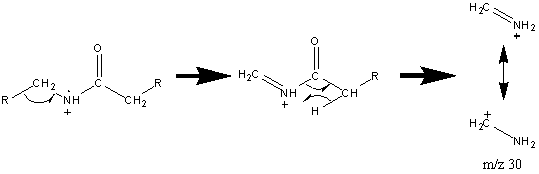
When the alkyl groups bonded to the nitrogen are longer than two carbons, another rearrangement is discernable.
Aromatic amides have a more prominent molecular ion peak. The common fragments are characterized by the loss of NR2 to form a resonance stabilized cation followed by the subsequent loss of CO.
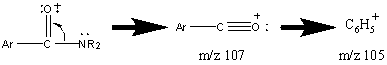
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008