7.2.4 Electron (hard) versus Chemical (soft) Ionization.
As noted in Chapter 1, the most common form of ionization in MS is electron ionization (EI) that is considered a hard source since is creates numerous fragments and allows for a unique fragmentation pattern. Several spectral libraries and computer search/match routines are available to aid in analyte identification. In contrast, chemical ionization (CI) is a milder form of ionization. Chemical ionization is rarely used for fragmentation pattern recognition, but is used to observe or obtain the molecular mass of the molecular ion. This experiment shows the electron and chemical ionization of three compounds.
EXPERIMENTAL PROCEDURES:
Chemicals and Supplies:
-A 25 ppm solution of 2,2’,6’6,-tetrachlorobiphenyl in isooctane
-A 50 ppm solution of cyclohexanol in methanol
-A 50 ppm solution of decanoic acid methyl ester in methanol
GC-MS Settings:
-Capillary Column: DB-5: Poly(phenylmethyldimethyl) siloxane (5 % phenyl)
30 m x 0.25 mm; 0.25 mm phase coating
-Injection Volume: 1.00 mL
-Splitless Injection for: 0.50 min.
-Split Flow Rate: 50 mL/min.
-Column Flow: 1.2 mL/min.
-Linear Velocity: 40 cm/s
-Injector Temperature: 250 C
- MS Transfer Line: 250 C
-Detector Temperature: 230 C
-Quadrupole Temperature: 150-250 C
-Oven Program: 55 C and hold for zero minutes, 5 C to 250 C, hold for ten minutes
-Total Run Time: 49 min.
Procedures:
Inject the standard solutions and analyze them using the instrument conditions given above.
RESULTS:
Spectra of the three compounds for EI and CI are shown below.
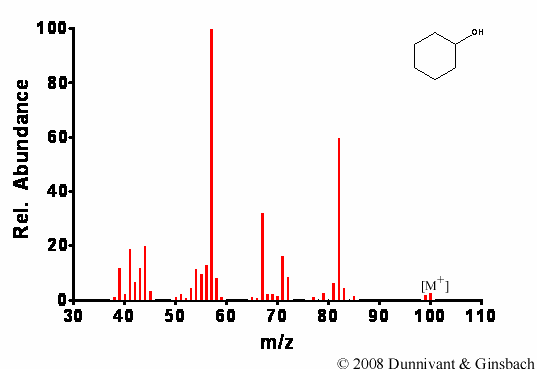
Figure 7.2. Fragmentation of Cyclohexanol by EI.
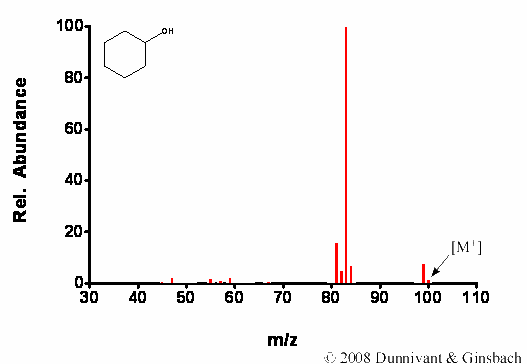
Figure 7.3. Fragmentation of Cyclohexanol by CI.
First, note the presence of the molecular ion using both ionization techniques. As expected, extensive fragmentation of cyclohexanol occurs for the EI analysis and follows the rules for fragmentation of alcohols given in Chapter 6, while minor fragmentation occurs in the CI analysis.
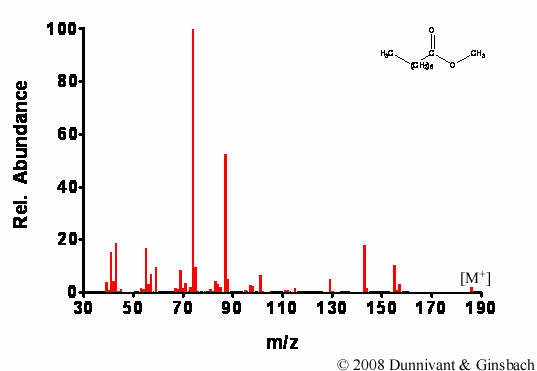
Figure 7.4. Fragmentation of Decanoic Acid Methyl Ester by EI.
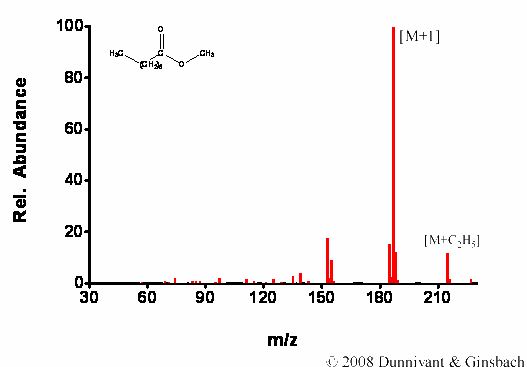
Figure 7.5. Fragmentation of Decanoic Acid Methyl Ester by CI.
Similar results are found for decanoic acid methyl ester; extensive fragmentation occurs during EI, but not during CI. Furthermore, for CI the molecular ion is more pronounced and the M+C2H5 ion is present in significant concentrations.
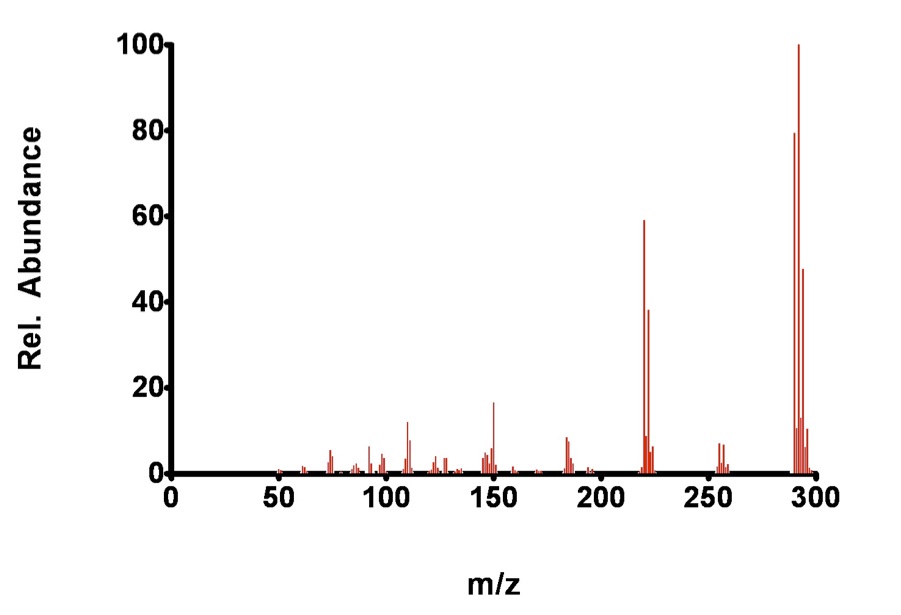
Figure 7.6. Fragmentation of 2,2’,6,6’-tetrachlorobiphenyl by EI.
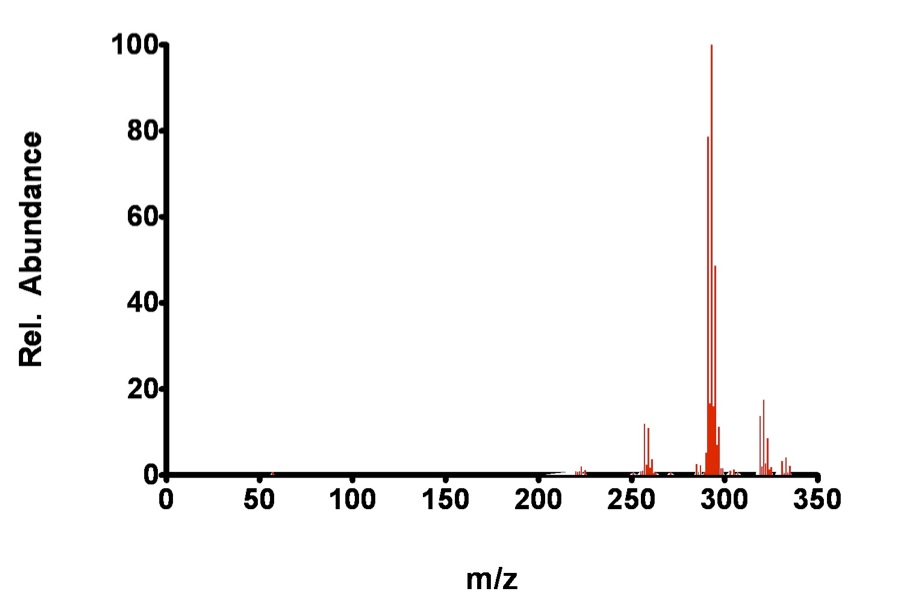
Figure 7.7. Fragmentation of 2,2’,6,6’-tetrachlorobiphenyl by CI.
The analyte, 2,2’,6,6’-TCB is so stable, even under the conditions in the EI chamber that the molecular ion is still a dominant peak. Again, some fragmentation occurs during EI, while additions are observed for the CI technique.
When to use EI and CI: Most MS analysis uses EI because it yields easily identified (via a fragmentation library) and unique fragmentation patterns. However, CI is used in two main cases: (1) when the point of the analysis is to obtain information about the molecular weight of the molecular ion and (2) when a better (lower) detection limit can be obtained using CI. Chemical ionization can be used in two modes, positive and negative.
As noted in section 1.5.1.2b: Chemical ionization is most commonly used to create positive ions, but some analytes, such as those containing acidic groups or electronegative elements (i.e. chlorinated hydrocarbons) will also produce negative ions that can be detected by reversing the polarity on the accelerator and detector. Some of these analytes produce superior detection limits with CI as opposed to EI, while others only give increased sensitivity (slope of the response to concentration line). Negative ions are produced by the capture of thermal electrons (relatively slower electrons with less energy than those common in the electron beam) by the analyte molecule. Thermal electrons are present from the low energy end of the distribution of electrons produced by the lower-energy CI source (~20 eV as opposed to ~70 eV in EI). These low energy electrons arise mostly from the chemical ionization process but also from analyte/electron collisions.”
| Frank's Homepage |
©Dunnivant & Ginsbach, 2008